Summary
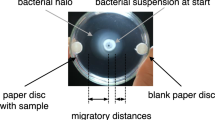
Root exudate from seedlings ofCicer arietinum L. was collected in a chamber under aseptic conditions. The exudate was fractionated into anion, cation and neutral fractions. The anionic fraction was made up of galacturonic acid, gluconic acid, mannuronic acid and two unidentified compounds withR f values 0.56 and 0.62. The cationic fraction contained alanine, arginine, aspartic acid, cystine, glycine, histidine, isoleucine, leucine, lysine and serine. The neutral fraction was made up of arabinose, galactose, glucose, ribose and xylose. The amino acids contributed to the bulk of the root exudate. The ratio of anionic, cationic and neutral fraction was 1∶7∶2. The crude root exudate was tested for its chemotactic ability using the capillary tube method. It was highly chemotactic for theRhizobium sp. The individual fractions and their various combinations were tested for chemotaxis. The chemotactic response of the Cicer strain of Rhizobium was least with anionic fraction most with cationic fraction and intermediate with neutral fraction. Maximum chemotactic response among the fractional combinations was obtained with all the three fractions and least with cationic plus neutral factions. Individual compounds constituting the various fractions were also tried for their ability to elicit chemotactic response. The organism exhibited maximum positive chemotactic response to histidine and negative response to alanine among the amino acids and to glucose and gluconic acid among the sugars and sugar acids.
Similar content being viewed by others
References
Adler, J., A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis ofEscherichia coli. J. Gen. Microbiol.74, 77–91 (1972).
Chailakbyan, M. and Megrabyan, A. A., Root secretions of leguminous plants and their effect on the growth of root nodule bacteria. Izvest. Akad. Nauk. Armyan S.S.R. Biol. i, Solzkokhoz Nauti.11, 7–12 (1958).
Currier, W. W. and Strobel, G. A., Chemotaxis ofRhizobium spp. to plant root exudates. Plant Physiol.57, 820–823 (1976).
Egeraat, A. W. S. M. van., Pea root exudates and their effect upon root nodule bacteria. Meded. Landbouwhogesch. Wageningen, Nederland (1972).
Egeraat, A. W. S. M. van., The positive role of homoserine in the development ofRhizobium leguminasarum in the rhizosphere of pea seedlings. Plant and Soil42, 381–386 (1975).
Gitte, R. R., Rai, P. V. and Patil, R. B. Chemotaxis ofRhizobium sp. towards root exudates ofCicer arictinum Linn. Indian J. Microbiol. 17th Ann. Conf. pp. 8 (abst-1976).
Hazelbauer, G. L., Mesibov, R. E., and Adler, J.,Eschcrichia coli mutants defective in chemotaxis towards specific chemicals. Proc. Nat. Acad. Sci (Wash)64, 1300–1307 (1969).
Kandasamy, D., Kesavan, R., Ramasamy, K. and Prasad, N. N., Occurrence of microbial inhibitors in the exudate of certain leguminous seeds. Indian J. Microbiol.,14, 25–30 (1974).
Nelson, N., A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem.158, 375–380 (1944).
Peters, B. J. and Alexander, M., Effect of legume exudates on the root nodule bacteria. Soil Sci.102, 380–387 (1966).
Rai, P. V. and Strobel, G. A., Chemotaxis of zoospores ofAphanomyces cochlioides to sugarbeet seedlings. Phytopathology56, 1365–1369 (1966).
Rovira, A. D.,Rhizobium numbers in the rhizosphere of red clover and paspalum in relation to soil treatment and the numbers of bacteria and fungi. Australian J. Agric. Res.12, 77 (1961).
Seymour, F. W. K. and Doetsch, N., Chemostatic response by motile bacteria. J. Gen. Microbiol.78, 287–296 (1973).
Timonin, M. I., Peterson, E. A., and Rouatt, J. W., Effects of amino acids and substances from wheat roots on the soil borne plant pathogenCochliobolus sativus. Soil Sci.118, 180–185 (1974).
Trevelyan, W. E., Procter, D. P. and Harrison, J. S., 1950. Detection of sugars on paper chromatograms. Nature166, 444–445 (1950).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gitte, R.R., Rai, P.V. & Patil, R.B. Chemotaxis ofRhizobium sp. Towards root exudate ofCicer Arietinum L.. Plant Soil 50, 553–566 (1978). https://doi.org/10.1007/BF02107208
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02107208