Abstract
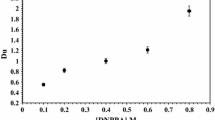
Liquid-liquid extraction of uranium (VI) from aqueous phosphoric acid solution by triisodecylamine (Alamine 310), tri-n-butyl phosphate (TBP), di-n-pentyl sulfoxide (DPSO) and their mixtures in benzene in the range 1–10M aqueous H3PO4 shows that extraction is maximum (≈80%) in the higher acidity range 6–8 M. Extraction of this metal ion by bis(2,4,4-trimethylpentyl)phosphinicacid (Cyanex 301) and its mixtures studied in the range 0.2–1.0M aqueous H3PO4 is far from being quantitative. Antagonism in extraction by mixtures of extractants is observed in most of the cases. Extraction of molybdenum(VI) under identical conditions shows that it is quantitative in the lower acidity range upto 2M H3PO4. Separation of uranium(VI) from molybdenum(VI) is feasible by Alamine 310, TBP and DPSO, the order of efficiency being TBP>DPSO>Alamine 310.
Similar content being viewed by others
References
S. STENSTROM, G. ALY, Hydrometallurgy, 14 (1985) 231.
R. FITOUSSI, C. MUSIKAS, Sep. Sci. Technol., 15 (1980) 845.
P. A. NOIROT, M. WOZNIAK, Hydrometallurgy, 13 (1985) 229.
I. BRCIC, I. FATOVIC, S. MELES, E. POLLA, M. RADOSEVIC, Hydrometallurgy, 18 (1987) 117.
G. MOUYSSET, J. MOLINER, M. LENZI, Hydrometallurgy, 11 (1983) 165.
W. J. MAECK, M. E. KUSSY, J. E. REIN, Annal. Chem., 33 (1961) 237.
E. M. SCADDEN, Nucleonics, 15 (1957) 102.
R. N. MOHANTY, S. SINGH, V. CHAKRAVORTTY, K. C. DASH, J. Radioanal. Nucl. Chem., 132 (1989) 359.
R. N. MOHANTY, S. SINGH, V. CHAKRAVORTTY, K. C. DASH, J. Radional. Nucl. Chem., 152 (1991) 21.
H. GORECKI, K. GRABAS, H. GORECKA, Pr. Nauk. Inst. Technol. Nieorg Nawozow Miner. Politech. Wroclaw, 29 (1985) 159; C.A., 105 (1986) 82875n.
J. YOU, Z. ZHOU, Q. QIN, He Huaxue Yu Fangshe Huaxue, 10 (1988) 207; C. A., 110 (1989) 219961n.
W. A. RICKELTON, Solvent Extr. Ion Exch., 6 (1988) 1173.
Y. SHIGETOMI, T. KOJIMA, I. NOUMA, S. HIRONO, Okiayima Rika Daigaku Kiyo, A, 20 (1985) 19; C.A., 103 (1985) 199011c.
H. M. NALLAH, H. LATROUS, Rev. Fac. Sci., Tunis, 2 (1982) 81; C.A., 100 (1984) 10476g.
K. W. KIM, J. H. YOO, H. S. PARK, Hwahak Konghak, 24 (1986) 171; C.A., 105 (1986) 212249z.
H. M. CHEN, H. J. CHEN, Y. M. TSAI, T. W. LEE, G. TING, Chem. Sep. Dev. Sel. Pap. Int. Conf. Sep. Sci. Technol., 2 (1986) 397.
C. H. KIM, G. S. CHUNG, H. B. YANG, J. H. KIM, Report 1982, KAERI/RR-292-81; C.A., 98 (1983) 78984s.
S. R. MOHANTY, A. S. REDDY, J. Inorg. Nucl. Chem., 37 (1975) 191, 1977.
A. I. BUSEV, V. G. TIPTSOVA, V. M. IVANOV, Analytical Chemistry of Rare Elements, MIR Publishers, Moscow, 1983.
A. I. VOGEL, A Textbook of Quantitative Inorganic Analysis, 3rd Ed., Longman, London, 1971.
F. T. BUNUS, V. C. DOMOCOS, P. DUMITRESCU, J. Inorg. Nucl. Chem., 40 (1978) 117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Behera, P., Mishra, R. & Chakravortty, V. Solvent extraction of uranium(VI) and molybdenium(VI) by Alamine 310 and its mixtures from aqueous H3PO4 solution. Journal of Radioanalytical and Nuclear Chemistry, Articles 173, 161–169 (1993). https://doi.org/10.1007/BF02102708
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02102708