Abstract
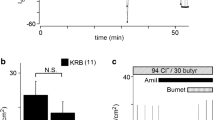
It has been shown that feces of patients with ulcerative colitis uniformly contain sulfate reducing bacteria. Sulfide produced by these bacteria interferes with butyrate-dependent energy metabolism of cultured colonocytes and may be involved in the pathogenesis of ulcerative colitis. Mucosal biopsies from the sigmoid rectum of 10 patients (no caner, polyps, inflammatory bowel disease) were incubated with either NaCl, sodium hydrogen sulfide (1 mmol/L), a combination of both sodium hydrogen sulfide and butyrate (10 mmol/L), or butyrate. Mucosal proliferation was assessed by bromodeoxyuridine labeling of cells in S-phase. Compared to NaCl, sulfide increased the labeling of the entire crypt significantly, by 19% (p < 0.05). This effect was due to an expansion of the proliferative zone to the upper crypt (compartments 3–5), where the increase in proliferation was 54%. Sulfide-induced hyperproliferation was reversed when samples were coincubated with sulfide and butyrate. The study shows that sodium hydrogen sulfide induces mucosal hyperproliferation. Our data support a possible role of sulfide in the pathogenesis of UC and confirm the role of butyrate in the regulation of colonic proliferation and in the treatment of UC.
Similar content being viewed by others
References
Beerens H, Romond C: Sulfate reducing anaerobic bacteria in human feces. Am J Clin Nutr 30:1770–1779, 1977
Gibson GR, Macfarlane GT, Cummings JH: Occurrence of sulphate reducing bacteria in human feces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol 65:103–111, 1988
Gibson GR, Macfarlane S, Macfarlane GT: Metabolic interactions involving sulphate reducing and methanogenic bacteria in the large intestine. FEMS Microbiol Ecol 12:117–123, 1993
Postgate JR: The Sulphate Reducing Bacteria, 2nd ed. Cambridge, UK, Cambridge University Press, 1984
Florin THJ, Gibson GR, Neale G, Cummings JH: A role of sulfate reducing bacteria in ulcerative colitis. Gastroenterology 98:A170, 1990
Gibson GR, Cummings JH, Macfarlane GT: Growth and activities of sulphate reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Ecol 86:103–112, 1991
Gibson GR, Cummings JH, Macfarlane GT, Allison C, Segal J, Vorster HH, Walker ARP: Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut 31:679–683, 1990
Gossel TA, Bricker JD: Principles of Clinical Toxicology. New York, Raven, 1990
Weiseger RA, Pinkus LM, Jacoby WB: Thiol S-methyltransferase: Suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol 29:2885–2887, 1980
Marcus R, Watt J: Seaweeds and ulcerative colitis in laboratory animals. Lancet 2:489, 1969
Kitano A, Matsumoto T, Hiki M, Hashimura H, Yoshihasu K, Okawa K, Kuwajiama S, Kobayashi K: Epithelial dysplasia of the rabbit colon induced by degraded carrageenan. Cancer Res 46:1374–1376, 1986
Yamada M, Okusha T, Okayasu I: Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextrane sulphate sodium. Gut 33:1521–1527, 1992
Roediger WEW, Duncan A, Kapaniris O, Millard S: Reducing sulfur compounds of the colon impair colonocyte nutrition: Implications for ulcerative colitis. Gastroenterology 104:802–809, 1993
Roediger WEW, Duncan A, Lapaniris O, Millard S: Sulphide impairment of sulphide oxidation in rat colonocytes: A biochemical basis for ulcerative colitis? Clin Sci 85:623–627, 1993
Roediger WEW: Utilization of nutrients by isolated cells of the rat colon. Gastroenterology 83:424–429, 1982
Whitehead RH, Young GP, Bhathal PS: Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut 27:1457, 1986
Kim YS, Tsao D, Siddiqui B, Whitehead JS, Arnstein P, Bennett J, Hicks J: Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer 45:1185–1192, 1980
Harig JM, Soergel KH, Komorowski RA, Wood CM: Treatment of diversion colitis with short chain fatty acid irrigation. N Engl J Med 320:23–26, 1989
Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H: Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 103:51–56, 1992
Breuer RI, Buto SK, Christ ML, Bean J, Vernia P, Paoluzi P, Di Polo MC, Caprilli R: Rectal irrigation with short chain fatty acids for distal ulcerative colitis. Dig Dis Csi 36:185–192, 1991
Gibson PR, Vam de Pol E, Maxwell LE, Gabriel A, Doe WF: Isolation of colonic crypts that maintain structural and metabolic viability in vitro. Gastroenterology 96:283–291, 1989
Bartram HP, Scheppach W, Schmid H, Hofmann A, Dusel G, Richter F, Richter A, Kasper H: Proliferation of human colonic mucosa as an intermediate biomarker of carcinogenesis: Effects of butyrate, deoxycholate, calcium, ammonia, and pH. Cancer Res 53:3283–3288, 1993
Lipkin M: Biomarkers of increased susceptibility to gastrointestinal cancer: New application to studies of cancer prevention in human subjects. Cancer Res 48:235–245, 1988
Lipkin M: Application of intermediate biomarkers to studies of cancer prevention in the gastrointestinal tract: Introduction and perspective. Am J Clin Nutr 54:188S-192S, 1991
Scheppach W, Bartram P, Richter A, Richter F, Liepold H, Dusel G, Hofstetter G, Ruthlein J, Kasper H: Effect of short-chain fatty acids on the human colonic mucosa in vitro. J Parent Enteral Nutr 16:43–48, 1992
Moore WEC, Johnsos JL, Holdeman LV: Emendation of Bacteroidaceae and Butyvibrio and descriptions of Desulfomonas gen. nov. and ten new species in the genera Desulfomonas, Butyvibrio, Eubacterium, Clostridum and Ruminococcus. Int J Syst Microbiol 26:238–246, 1976
Christl SU, Gibson GR, Cummings JH: Role of dietary sulfate in the regulation of methanogenesis in the human large intestine. Gut 33:1234–1238, 1992
Florin THJ, Neale G, Gibson GR, Christl SU, Cummings JH: Metabolism of dietary sulphate: Absorption and excretion in humans. Gut 32:766–773, 1991
Vercellotti JR, Salyers AA, Bullard WS, Wilkins S: Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem 55:1190–1196, 1977
Gibson GR, Cummings JH, Macfarlane GT: Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol 54:2750–2755, 1988
Macfarlane GT, Gibson GR, Cummings JH: Comparison of fermentation reactions in different regions of the colon. J Appl Bacteriol 72:57–64, 1992
Watt J, Marcus R: Ulcerative colitis in rats fed degraded carrageenan. J Pharm Pharmacol 22:130–131, 1970
Onderdonk AB, Hermos JA, Bartlett JG: The role of the intestinal microflora in experimental colitis. Am J Clin Nutr 30:1819–1825, 1977
Onderdonk AB, Hermos JA, Dzink JL, Bartlett JG: Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology 74:521–527, 1978
Roediger WEW, Babidge W, Millard S: Methionine derivatives diminish sulphide damage to colonocytes—implication for ulcerative colitis. Gut 39:77–81, 1996
Cummings JH: Fermentation in the human large intestine: Evidence and implications for health. Lancet 1:1206–1209, 1983
Macfarlane GT, Cummings JH: The colonic flora, fermentation, and large bowel digestive function.In The Large Intestine: Physiology, Pathophysiology, and Disease, S Philips, JH Pemberton, RG Shorter (eds). New York, Raven Press, 1991, p 51
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christl, S.U., Eisner, HD., Dusel, G. et al. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa. Digest Dis Sci 41, 2477–2481 (1996). https://doi.org/10.1007/BF02100146
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02100146