Summary
1. While intracellular calcium concentrations are closely regulated, two types of ion channels in neurons allow calcium influx: both voltage-activated and NMDA-activated channels are significantly permeable to calcium. In this study we compare the effects of lead (Pb2+) on currents carried through voltage-activated calcium channels and NMDA-activated channels.
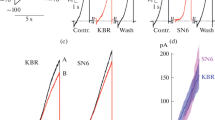
2. Pb2+ reduces voltage-activated calcium channel currents elicited by a voltage jump from −80 to 0 mV at 0.1 to 1 µM, with an IC50 of 0.64 µM and a Hill slope of 1.22. This effect was partially reversible and not voltage dependent. Sodium and potassium currents were relatively unaffected at Pb2+ concentrations sufficient to block calcium channel currents by more than 80%. Pb2+ is, thus, a potent, reversible and selective blocker of voltage-dependent calcium channel currents.
3. A fast reversible and slow irreversible blocking action of Pb2+ was found on NMDA-activated currents. When Pb2+ was applied simultaneously with aspartate and glycine (Asp/Gly), the inward currents were rapidly and reversibly reduced in a dose-dependent manner with a minimum effective concentration below 2 µM and a total blockade (>80%) with 100 µM Pb2+. The IC50 was ∼45 µM and the Hill coefficient 1.1. Preincubation with 50 µM Pb2+ resulted in a greater reduction in the response to Asp/Gly/Pb2+. This effect was reversed within 2 to 5 sec of wash. The lack of voltage dependence suggests that Pb2+ does not block the channel but rather alters the binding of agonists. Prolonged superfusion of a cell with the Asp/Gly/Pb2+-containing external solution resulted in a slow and irreversible decrease in the Asp/Gly activated current. No clear threshold concentration is found for this slow and irreversible effect of Pb2+. This slow action might be more important for neurotoxic effects of Pb2+.
Similar content being viewed by others
References
Alkondon, M., Costa, A. C., Radhakrishnan, V., Aronstam, R. S., and Albuquerque, E. X. (1990). Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead.FEBS Lett. 261:124–130.
Altmann, L., Sveinsson, K., and Wiegand, H. (1991). Long-term potentiation in rat hippocampal slices is impaired following acute lead perfusion.Neurosci. Lett. 128:109–112.
Altmann, L., Weinsberg, F., Sveinsson, K., Lilienthal, H., Wiegand, H., and Winneke, G. (1993). Impairment of long-term potentiation and learning following chronic lead exposure.Toxicol. Lett. 66:105–112.
Audesirk, G., and Audesirk, T. (1991). Effects of inorganic lead on voltage sensitive calcium channels in NIE-115 neuroblastoma cells.Neurotoxicology 12:519–528.
Benetou Marantidou, A., Nakou, S., and Micheloyannis, J. (1988). Neurobehavioral estimation of children with life-long increased lead exposure.Arch. Environ. Health 43:392–395.
Boland, L. M., and Dingledine, R. (1990). Multiple components of both transient and sustained barium currents in a rat dorsal root ganglion cell line.J. Physiol. Lond. 420:223–245.
Brennan, R. J. W., and Cantrill, R. C. (1979). d-Aminolevulinic acid is a potent agonist for GABA receptors.Nature 280:514–515.
Büsselberg, D., Evans, M. L., Rahmann, H., and Carpenter, D. O. (1991a). Effects of inorganic and triethyl lead and inorganic mercury on the voltage-activated calcium channel of Aplysia neurons.Neurotoxicology 12:733–744.
Büsselberg, D., Evans, M. L., Rahmann, H., and Carpenter, D. O. (1991b). Lead and zinc block a voltage-activated calcium channel of Aplysia neurons.J. Neurophysiol. 65:786–795.
Büsselberg, D., Evans, M. L., Haas, H. L., and Carpenter, D. O. (1993). Blockade of mammalian and invertebrate calcium channels by lead.Neurotoxicology 14:249–258.
Büsselberg, D., Platt, B., Michael, D., Carpenter, D. O., and Haas, H. L. (1994a). Mammalian voltage-activated calcium channel currents are blocked by Pb2+, Zn2+, and Al3+.J. Neurophys. 71:1491–1497.
Büsselberg, D., Domann, R., Wunder, L., and Haas, H. L. (1994b). Lead reduces calcium entry without passing the cell membrane of mammalian neurons: Fura 2 measurementsNeurosci. Abstr. 673:21.
Carroll, P. T., Silbergeld, E. K., and Goldberg, A. M. (1977). Alteration of central cholinergic function by chronic lead acetate exposure.Biochem. Pharmacol. 26:397–402.
Chad, J. E., and Eckert, R. (1986). An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones.J. Physiol. Lond. 378:31–51.
Chizhmakov, I. V., Kiskin, N. I., Krishal, O. A., and Tsyndrenko, A. Y. (1989). Glycine action on NMDA receptors in rat hippocampal neurons.Neurosci. Lett. 99:131–136.
Christine, C. W., and Choi, D. W. (1990). Effects of zinc on NMDA-receptor mediated channel currents in cortical neurons.J. Neurosci. 10:108–116.
Davis, S., Butcher, S. P., and Morris, R. G. (1992). The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro.J. Neurosci. 12:21–34.
Evans, M. L., Büsselberg, D., and Carpenter, D. O. (1991). Pb2− blocks calcium currents of cultured dorsal root ganglion cells.Neurosci. Lett. 129:103–106.
Fox, A. P., Nowycky, M. C., and Tsien, R. W. (1987a). Single-channel recordings of three types of calcium channels in chick sensory neurones.J. Physiol. Lond. 394:173–200.
Fox, A. P., Nowycky, M. C., and Tsien, R. W. (1987b). Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones.J. Physiol. Lond. 394:149–172.
Harris, E. W., Ganong, A. H., and Cotman, C. W. (1984). Longterm potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors.Brain Res. 323:132–137.
Hernberg, S., and Nikkanen, J. (1970). Enzyme inhibition by lead under normal urban conditions.Lancet 1:63–64.
Hernberg, S., Viekko, E., and Hasan, L. (1967). Red cell membrane ATPase in workers exposed to inorganic lead.Arch. Environ. Health 14:319–324.
Holtzman, D., and Hsu, J. S. (1976). Early effects of inorganic lead on immature rat brain mitochondrial respiration.Pediatr. Res. 10:70–75.
Hori, N., Büsselberg, D., Matthews, M. R., Parsons, P. J., and Carpenter, D. O. (1993). Lead blocks LTP by an action not at NMDA receptors.Exp. Neurol. 119:192–197.
Lansman, J. B., Hess, P., and Tsien, R. W. (1986). Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore.J. Gen. Physiol. 88:321–347.
Legendre, P., and Westbrook, G. L. (1990). The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones.J. Physiol. Lond. 429:429–449.
Leviton, A., Bellinger, D., Allred, E. N., Rabinowitz, M., Needleman, H., and Schoenbaum, S. (1993). Pre- and postnatal low-level lead exposure and children's dysfunction in school.Environ. Res. 60:30–43.
Linás, R., Sugimori, M., Hillman, D. E., and Cherksey, B. (1992). Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system.Trends Neurosci. 15:351–355.
Markovac, J., and Goldstein, G. W. (1988). Lead activates protein kinase C in immature rat brain microvessels.Toxicol. Appl. Pharmacol. 96:14–23.
Mayer, M. L., and Westbrook, G. L. (1987). Permeation and block of N-methyl-aspartic acid receptor channels by divalent cations in mouse cultured central neurons.J. Physiol. Lond. 394:501–527.
McMichael, A. J., Baghurst, P. A., Wigg, N. R., Vimpani, G. V., Robertson, E. F., and Roberts, R. J. (1988). Port Pirie Cohort Study: Environmental exposure to lead and children's abilities at the age of four years.N. Engl. J. Med. 319:468–475.
Mintz, I. M., Adams, M. E., and Bean, B. P. (1992). P-type calcium channels in rat central and peripheral neurons.Neuron 9:85–95.
Mintz, I. M., Venema, V. J., Swiderek, K. M., Lee, T. D., Bean, B. P., and Adams, M. E. (1992). P-type calcium channels blocked by the spider toxin omega-Aga-IVA.Nature 355:827–829.
Murakami, K., Feng, G., and Chen, S. G. (1993). Inhibition of brain protein kinase C subtypes by lead.J. Pharmacol. Exp. Ther. 264:757–761.
Nathan, R. D., Kanai, K., Clark, R. B., and Giles, W. (1988). Selective block of calcium current by lanthanum in single bullfrog atrial cells.J. Gen. Physiol. 91:549–572.
Needleman, H. L., and Bellinger, D. (1991). The health effects of low level exposure to lead.Annu. Rev. Public Health 12:111–140.
Needleman, H. L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H., Maher, C., and Barrett, P. (1979). Deficits in psychologic and classroom performance of children with elevated dentine lead levels.N. Engl. J. Med. 300:689–695.
Oyama, Y., Nishi, K., Yatani, A., and Akaike, N. (1982). Zinc current in Helix soma membrane.Comp. Biochem. Physiol. C 72:403–410.
Padich, R. A., Dietrich, K. N., and Pearson, D. T. (1985). Attention, activity level, and lead exposure at 18 months.Environ. Res. 38:137–143.
Pekel, M., Platt, B., and Büsselberg, D. (1993). Mercury (Hg2−) decreases voltage-gated calcium channel currents in rat DRG and Aplysia neurons.Brain Res. 632:121–126.
Peters, S., Koh, J., and Choi, D. W. (1987). Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons.Science 236:589–593.
Piomelli, S., Seaman, C., and Zullow, D. (1982). Threshold for lead damage to heme synthesis in urban children.Proc. Natl. Acad. Sci. USA 79:3335–3339.
Platt, B., Haas, H. L., and Büsselberg, D. (1993). Extracellular pH modulates aluminum-blockade of mammalian voltage-activated calcium channel currents.NeuroReport 4:1251–1254.
Sather, W., Dieudonne, S., Macdonald, J., and Ascher, P. (1992). Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurons.J. Physiol. Lond. 450:643–672.
Scroggs, R. S., and Fox, A. P. (1992). Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size.J. Physiol. Lond. 445:639–658.
Simons, T. J. (1988). Calcium and neuronal function.Neurosurg. Rev. 11:119–129.
Simons, T. J., and Pocock, G. (1987). Lead enters bovine adrenal medullary cells through calcium channels.J. Neurochem. 48:383–389.
Swandulla, D., and Armstrong, C. M. (1989). Calcium channel block by cadmium in chicken sensory neurons.Proc. Natl. Acad. Sci. USA 86:1736–1740.
Tomsig, J. L., and Suszkiw, J. B. (1990). Pb2+-induced secretion from bovine chromaffin cells: fura-2 as a probe for Pb2+.Am. J. Physiol. 259:762–768.
Tsien, R. W., Hess, P., McCleskey, E. W., and Rosenberg, R. L. (1987). Calcium channel mechanisms of selectivity, permeation, and block.Annu. Rev. Biophys. Biophys. Chem. 16:265–290.
Ujihara, H., and Albuquerque, E. X. (1992). Developmental change of the inhibition by lead of NMDA-activated currents in cultured hippocampal neurons.J. Pharmacol. Exp. Ther. 263:868–875.
Uteshev, V., Büsselberg, D., and Haas, H. L. (1993). Pb2+ modulates the NMDA-receptor-channel complex.Naunyn Schmiedebergs Arch. Pharmacol. 347:209–213.
Westbrook, G. L., and Mayer, M. L. (1987). Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons.Nature 328:640–643.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Büsselberg, D., Michael, D. & Platt, B. Pb2+ reduces voltage- andN-methyl-d-aspartate (NMDA)-activated calcium channel currents. Cell Mol Neurobiol 14, 711–722 (1994). https://doi.org/10.1007/BF02088679
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02088679