Abstract
Purpose: The aim of this study was to compare the development of bovine parthenogenetic and in vitro fertilization-derived (IVF) embryos. Oocytes were matured, fertilized, or allowed to activate spontaneously by aging and then cultured for up to 14 days in vitro. Cleavage and development rates, morphology, ultrastructure, and transcriptional activity were compared.
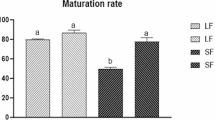
Results: Very few parthenogenotes (7.5% of aged oocytes) were obtained and none developed to the hatched blastocyst stage. The pattern of3H-uridine incorporation at the two-, four-, and eight-cell stages was similar in both types of embryos. Morphological study, however, revealed that none of the parthenogenotes showed features associated with long-term development: they were developmentally delayed and usually arrested before reaching the blastocyst stage (only 0.5% of aged oocytes developed to the blastocyst stage). Structural differences between parthenogenotes and the IVF-derived embryos were observed at all stages of development.
Conclusions: Structural observation suggested that failure of long-term development of bovine parthenogenotes may have a metabolic basis. Some functional changes appeared to coincide with chronological age rather than developmental age. Even though development of parthenogenotes was limited, some features of nuclear maturation and activation of the embryonic genome occur without contribution of the paternal genome.
Similar content being viewed by others
References
Kaufman MH: Parthenogenesis: A system facilitating understanding of factors that influence early mammalian development. Vol 1.In Progress in anatomy. RJ Harrison, RL Holmes (eds). Cambridge, Cambridge University Press, 1981, pp 1–34
Whittingham DG: Parthenogenesis in mammals. Ox Rev Reprod Biol 1980;2:205–231
Stevens LC, Varnum DS, Eicher EM: Viable chimaeras produced from normal and parthenogenetic mouse embryos. Nature 1977;269:515–517
Stevens LC: Totipotent cells of parthenogenetic origin in a chimaeric mouse. Nature 1978;276:266–267
Anderegg C, Markert CL: Successful rescue of microsurgically produced homozygous uniparental mouse embryos via production of aggregation chimeras. Proc Natl Acad Sci USA 1986;83:6509–6513
Nagy A, Sass M, Markkula M: Systematic non-uniform distribution of parthenogenetic cells in adult mouse chimaeras. Development 1989;106:321–324
Paldi A, Nagy A, Markkula M, Barna I, Dezso L: Postnatal development of parthenogenetic-fertilized mouse aggregation chimeras. Development 1989;105:115–118
Monk M: Genomic imprinting. Genes Dev 1988;2:921–925
Solter D: Differential imprinting and expression of maternal and paternal genomes. Annu Rev Genet 1988;22:127–146
Surani MA, Allen ND, Barton SC, Fundele R, Howlett SK, Norris ML, Reik W: Developmental consequences of imprinting of parental chromosomes by DNA methylation. Phil Trans R Soc Lond B 1990;326:313–327
Pickering SJ, Johnson MH, Braude PR, Houliston E: Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod 1988;3:978–989
Taylor AS, Brande PR: The early development and DNA content of activated human oocytes and parthenogenetic human embryos. Hum Reprod 1994;9:2389–2397
Winston N, Johnson M, Pickering S, Braude P: Parthenogenetic activation and development of fresh and aged human oocytes. Fertil Steril 1991;56:904–912
Xu KP, Greve T, Smith S, Liehman P, Callesen H, Hyttel P: Parthenogenetic activation of cattle oocytes matured in vitro and cultured in rabbit oviducts. Theriogenology 1986;25:218
King WA, Xu KP, Sirard M-A, Leclerc P, Lambert RD, Jacques P: Cytogenetic study of parthenogenetically activated bovine oocytes matured in vivo and in vitro. Gamete Res 1988;20:265–274
Ware CB, Barnes FL, Mailei-Laurila M, First NL: Age dependence of bovine oocyte activation. Gamete Res 1989;22:265–275
Sun FZ and Moor RM: Nuclear transplantation in mammalian eggs and embryos.In Current Topics in Developmental Biology, RA Pedersen, and GP Schatten (eds). New York, London, Tokyo, Toronto, Academic Press, 1995, Vol. 30, pp. 147–176
Boediono A, Suzuki T: Pregnancies after transfer of aggregated parthenogenetic bovine activated oocytes. Theriogenology 1994;41:166
Xu KP, Yadav BR, Rorie RW, Plante L, Betteridge KJ, King WA: Development and viability of bovine embryos derived from oocytes matured and fertilized in vitro and co-cultured with bovine oviducal epithelial cells. J Reprod Fert 1992;94:33–43
Greve T, Xu KP, Callesen H, Hyttel P: In-vivo development of in-vitro fertilized bovine oocytes matured in vivo and in vitro. J Vitro Fert Embryo Transfer 1987;4:281–285
Parrish JJ, Susko-Parrish JL, Leibfried-Ruthledge ML, Crister ES, Eyestone WH, First NL: Bovine in vitro fertilization with frozen thawed semen. Theriogenology 1986;25:591–600
Hyttel P, Madsen I: Rapid method to prepare mammalian oocytes and embryos for transmission electron microscopy. Acta Anat 1987;129:12–14
Plante L, Pollard JW, King WA: A comparison of two autoradiographic methods for detecting radiolabeled nucleic acids in embryos. Mol Reprod Dev 1992;33:141–148
King WA, Linares T, Gustavsson I, Bane A: A method for preparation of chromosomes from bovine zygotes and blastocysts. Vet Sci Comm 1979;3:51–56
Plante L, Plante C, Shepherd DL, King WA: Cleavage and3H-uridine incorporation in bovine embryos of high in vitro developmental potential. Mol Reprod Dev 1994;39:375–383
Plante L, King WA: Light and electron microscopic analysis of bovine embyros derived by in vitro and in vivo fertilization. J Assist Reprod Genet 1994;11:15–529
Chang MC: Natural occurrence and artificial induction of parthenogenetic cleavage of ferret ova. Anat Rec 1957;128:187–200
Elsden RP, Nelson LD, Seidel GE Jr: Superovulating cows with follicle stimulating hormone and pregnant mare's serum gonadotrophin. Theriogenology 1978;9:17–26
van Blerkom J: Developmental failure in human reproduction associated with preovulatory oogenesis and preimplantation embyrogenesis.In Ultrastructure of Human Gametogenesis and Early Embryogenesis, J van Blerkom, PM Motta (eds). Boston, Kluwer Academic, 1989, pp 125–180
Webb M, Howlett SK, Maro B: Parthenogenesis and cytoskeletal organization in ageing mouse eggs. J Embryol Exp Morphol 1986;95:131–145
Kaufman MH, Barton SC, Surani SC: Normal postimplantation development of mouse parthenogenetic embryos to the forelimb bud stage. Nature 1977;265:53–57
Kawarsky SJ, Hansen PJ, Stubbingss RB, Basrur PK, King WA: Influence of chromosomal make-up on growth rate of bovine embryos. Biol Reprod 1994;50 (Suppl 1):88
McGrath J, Solter D: Nucleocytoplasmic interactions in the mouse embryo. J Embryol Exp Morphol 1986;97 (Suppl):277–289
First NL, Barnes FL: Development of preimplantation mammalian embryos.In Development of Preimplantation Embryos and Their Development. New York, Alan R Liss, 1989, pp 151–170
Hansmann I, Gebauer J, Grimm T: Impaired gene activity for 18S and 28S rRNA in early embryonic development of mouse parthenogenones. Nature 1978;272:377–378
Tesarik J, Kopecny V: Nucleic acid synthesis and development of human male pronucleus. J Reprod Fert 1989;86:549–558
Ram PT, Schultz RM: Reporter gene expression in G2 of the 1-cell mouse embryo. Dev Biol 1993;156:552–556
Matsumoto K, Masayuki A, Nakagata N, Takahashi A, Takahashi Y, Miyata K: Onset of paternal gene activation in early mouse embryos fertilized with transgenic mouse sperm. Mol Reprod Dev 1994;39:136–140
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD: Molecular Biology of the Cell. New York, Garland, 1983, p 1146
Bozzola JJ, Russell LD: Electron Microscopy. Principles and Techniques for Biologists. Boston, Jones and Bartlett, 1992, p 542
El-Shershaby AM, Hinchliffe JR: Cell redundancy in the zonaintact preimplantation mouse blastocyst: A light and electron microscope study of dead cells and their fate. J Embryol Exp Morphol 1974;31:643–654
Hegele-Hartung C, Fischer B, Beier H: Development of preimplantation rabbit embryos after in-vitro culture and embryo transfer: An electron microscopic study. Anat Record 1988;220:31–42
Tarkowski A, Wroblewska J: Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol 1967;18:155–180
Ham AW: Histology. Philadelphia: Lippincott, 1974, p 1006
Bedford JM: Fertilization.In Germ Cells and Fertilization, Vol 1. C R Austin, RV Short (eds). Cambridge, Cambridge University Press, 1982, pp 128–163
Author information
Authors and Affiliations
Corresponding author
Additional information
A portion of this paper was presented as a poster at the International Embryo Transfer Society's Annual Meeting, Denver, 1993 [Plante L, King WA: Developmental potential of spontaneously activated bovine oocytes. Theriogenology 1993;39:268 (abstract).]
Rights and permissions
About this article
Cite this article
Plante, L., King, W.A. In vitro development of spontaneously activated bovine oocytes. J Assist Reprod Genet 13, 435–446 (1996). https://doi.org/10.1007/BF02066178
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02066178