Abstract
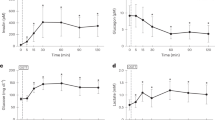
d-Lactic acidosis is seen in patients with intestinal bypass or short bowels in whom colonic producedd-lactate accumulates. An intestinal bypassed patient withd-lactic acidosis had higher fecald-lactate (122.4 mmol/liter) andl-lactate (90.1 mmol/liter) than described before in humans.d-Lactate fluctuated between 0.5 and 3.1 mmol/liter in plasma (normal<0.1 mmol/liter) and between 1.1 and 52.8 mmol/liter in urine (normal<0.7 mmol/liter) within a few hours, indicating that the human organism do metabolize and excreted-lactate. The patient withd-lactic acidosis had a 10-fold increasedDl-lactate production from glucose in fecal homogenates compared to 14 healthy controls and a patient with intestinal bypass, who did not haved-lactic acidosis. A 67% carbohydrate (starch)-enriched diet resulted in a minor elevation of fecal and plasma lactate, whereas 50 + 100+150 g of ingested lactose increasedd-lactate in feces (84.0 mmol/liter) and plasma (2.3 mmol/liter) considerably in the patient withd-lactic acidosis. Intestinal prolongation (22 cm ileum) had a temporary effect on fecal and plasmad-lactate, but intestinal continuity was reestablished 26 months later becaused-lactic acidosis recurred (plasma 8.6 mmol/liter, urine 101.3 mmol/liter). Large amounts of lactulose (160 g/day) to 12 normal individuals increasedd-lactate to 13.6±3.5 mmol/liter in feces, but never increasedd-lactate in plasma or urine. Thein vitro fermentation of glucose in fecal homogenates increasedDl-lactate, which disappeared after complete metabolization of the glucose.l-Lactate was converted tod-lactate andvice versa, and both were degraded to the short-chain fatty acids acetate, propionate, and butyrate. An infrequent, but elevated ability of the colonic flora to produce lactate may be a prerequisite ford-lactic acidosis to occur and may explain why the syndrome is so seldom seen even in patients with intestinal bypass or short bowels. The suggestion thatd-lactate is not metabolized and hence accumulates is probably not valid.
Similar content being viewed by others
References
Carr DB, Shih VE, Richter JM, Martin JB:d-Lactic acidosis simulating a hypothalamic syndrome after bowel bypass. Ann Neurol 11:195–197, 1982
Dahlquist NR, Perrault J, Wayne Callaway C, Jones JD:d-Lactic acidosis and encephalopathy after jejunoileostomy: Response to overfeeding and to fasting in humans. Mayo Clin Proc 59:141–145, 1984
Duran M, Van Biervliet JPGM, Kamerling JP, Wadman SK:d-Lactic aciduria, an inborn error of metabolism? Clin Chim Acta 74:297–300, 1977
Flourie B, Messing B, Bismuth E, Étanchaud F, Thuillier FJC: Acidosed lactique et encéphalopathie au cours d'un syndrome du syndrome du grêle court à l'occasion d'un traitement antibiotique. Gastroenterol Clin Biol 14:596–598, 1990
Haan E, Brown G, Bankier A, Mitchell D, Hunt S, Blakey J, Barnes G: Severe illness caused by the products of bacterial metabolism in a child with short gut. Eur J Pediatr 144:63–65, 1985
Halvorson J, Gale A, Lazarus C, Avioli LV:d-Lactic acidosis and other complications of intestinal bypass surgery. Arch Intern Med 144:357–360, 1984
Hudson M, Pocknee R, Mowat NA.d-Lactic acidosis in short bowel syndrome—an examination of possible mechanisms. Q J Med 74:157–163, 1990
Karton M, Rettmer RL, Lipkin E: Effect of parenteral nutrition and enteral feeding ond-lactic acidosis in a patient with short bowel. JPEN 11:586–589, 1987
Lipkin EW, Karton MA, Rettmer R, Chait A:d-Lactic acidosis in patients on long-term parenteral nutrition. JPEN 10:17S, 1986
Mason PD: Metabolic acidosis due tod-lactate. BMJ 292:1105–1106, 1986
Mayne AJ, Handy DJ, Preece MA, George RH, Booth IW: Dietary management ofd-lactic acidosis in short bowel syndrome. Arch Dis Child 65:229–231, 1990
Nghiem CH, Bui HD, Chaney RH: An unusual cause ofd-lactic acidosis. West J Med 148:332–334, 1988
Oh MS, Phelps K, Traube M, Barbosa-Saldivar JL, Boxhill C, Carroll H:d-Lactic acidosis in a man with short bowel syndrome. N Engl J Med 301:249–252, 1979
Perlmutter DH, Boyle JT, Campos JM, Egler JM, Watkins JB:d-Lactic acidosis in children: An unusual metabolic complication of small bowel resection. J Pediatr 102:234–238, 1983
Ramakrishnan T, Stokes P: Beneficial effects of fasting and low carbohydrate diet ind-lactic acidosis associated with short-bowel syndrome. JPEN 9:361–363, 1985
Rosenthal P, Pesce M: Long-term monitoring ofd-lactic acidosis in a child. J Pediatr Gastr Nutr 4:674–676, 1985
Satoh T, Narisawa K, Konno T, Katoh T, Fujiyama J, Tomoe A, Metoki K, Hayasaka K, Tada K, Ishibashi M, Yamane N, Mitsuoka T, Benno Y:d-Lactic acidosis in two patients with short bowel syndrome: Bacteriological analyses of the faecal flora. Eur J Pediatr 138:324–326, 1982
Schoorel EP, Giesberts MAH, Blom W, Van Gelderen HH:d-Lactic acidosis in a boy with short bowel syndrome. Arch Dis Child 55:810–812, 1980
Stolberg L, Rolfe R, Gitlin N, Merritt J, Mann L, Linder J, Finegold S:d-Lactic acidosis due to an abnormal gut flora. N Engl J Med 306:1344–1348, 1982
Traube M, Bock J, Boyer JL:d-Lactic acidosis after jejunoileal bypass: Identification of organic anions by nuclear magnetic resonance spectroscopy. Ann Intern Med 98:171–173, 1983
Editorial. The colon, the rumen, andd-lactic acidosis. Lancet 336:599–600, 1990
Dunlop RH, Hammond PB:d-Lactic acidosis of ruminants. Ann NY Acad Sci 119:1109–1152, 1965
Cross SA, Callaway CW:d-Lactic acidosis and selected cerebellar ataxia. Mayo Clin Proc 59:202–205, 1984
Mortensen PB, Hove H, Clausen MR, Holtug K: Fermentation of short-chain fatty acids and lactate in human faecal batch cultures. Scand J Gastroenterol 26:1285–1294, 1991
Gutmann I, Wahlefeld AW: Lactate determination with lactate dehydrogenase and NAD.In Methods of Enzymatic Analysis. HU Bergmeyer (ed). Academic Press, New York, 1974, pp 1464–1468
Zijlstra JB, Beukema J, Wolthers BG, Byrne BM, Groen A, Donkert L: Pretreatment methods prior to gas chromatographic analysis of volatile fatty acids from faecal samples. Clin Chim Acta 78:243–250, 1977
Bergmeyer HU, Bernt E, Schmidt F, Stork H:d-Glucose bestimmung mit hexokinase und glucose-6-phosphatdehydrogenase.In Methoden der Enzymatischen Analyse, Vol 2, 2nd ed, HU Bergmeyer (ed). U. Weinheim/bergstr.; Verlag Chemie 1970, pp 1163–1168
Ameen AZ, Powell GK, Jones LA: Quantitation of faecal carbohydrate excretion in patients with short bowel syndrome. Gastroenterology 92:493–500, 1987
Holtug K, Clausen MR, Hove H, Christiansen J, Mortensen PB: The colon in carbohydrate malabsorption: Short-chain fatty acids, pH, and osmotic diarrhoea. Scand J Gastroenterol 27:545–552, 1992
Roediger WEW: Fecal anions and lactate in severe ulcerative colitis. Dig Dis Sci 34:1801–1802, 1989 (letter).
Vernia P, Gnaedinger A, Hauck W, Breuer RI: Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci 33:1353–1358, 1988
Vernia P, Caprilli R, Latella G, Barbetti F, Magliocca FM, Cittadini M: Fecal lactate and ulcerative colitis. Gastroenterology 95:1564–1568, 1988
Kern F, Singleton JW, Struthers JE: Fecal lactic acid in carbohydrate malabsorption. J Lab Clin Med 64:874, 1964
Torres-Pinedo R, Lavastida M, Rivera CL, Rodriguez H, Ortiz A: Studies on infant diarrhea. Comparison of the effects of milk feeding and intravenous therapy upon the composition and volume of the stool and urine. J Clin Invest 43:469–480, 1966
Weijers HA, Van de Kamer JH, Dicke WK, Ijsseling J: Diarrhoea caused by deficiency of sugar splitting enzymes. Acta Paediatr 50:55–71, 1961
Thurn JR, Pierpont GL, Ludvigsen CW, Eckefeldt JH:d-Lactate encephalopathy. Am J Med 79:717–721, 1985
Cammack R: Assay, purification and properties of mammaliand-2-hydroxy acid dehydrogenase. Biochem J 115:55–64, 1969
Tubbs PK, Greville GD: The oxidation ofd-hydroxy acids in animal tissue. Biochem J 81:104–114, 1961
Tubbs PK: Effects of inhibitors on mitochondrial D-hydroxyacid dehydrogenase. Biochem J 82:36–42, 1962
Brandt RB, Waters MG, Rispler MJ, Kline ES:d-andl-lactate catabolism to CO2 in rat tissues. Proc Soc Exp Biol Med 175:328–335, 1984
Heute AK: Über die oxidation von D-laktat in der Rattenleber. Thesis. Munich, 1986
Giesecke D, Stangassinger M, Henle K:d(−) Milchsäure—ein stoffwechselproblem. Zeit Ernaehrungswiss 24:172–186, 1985
Connor H, Woods HF, Ledingham JGG: Comparison of the kinetics and utilisation ofd(−)- andl(+)-sodium lactate in normal man. Ann Nutr Metab 27:481–487, 1983
De Vrese M, Koppenhoefer B, Barth CA:d-Lactic acid metabolism after an oral load ofDl-lactate. Clin Nutr 9:23–28, 1990
Oh MS, Uribarri J, Alveranga D, Lazar I, Bazilinski N, Carroll H: Metabolic utilization and renal handling ofd-lactate in men. Metabolism 34:621–625, 1985
Christopher MM, Eckfeldt JH, Eaton JW: Propylene glycol ingestion causesd-lactic acidosis. Lab Invest 62:114–118, 1990
Thornalley PJ: Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 254:751–755, 1988
Kandler O: Carbohydrate metabolism in lactic acid bacteria. J Antonie Van Leeuwenhoek 49:209–224, 1983
Taguchi H, Ohta T:d-Lactate dehydrogenase is a member of thed-isomer-specific 2-hydroxyacid dehydrogenase family. J Biol Chem 266:12588–12594, 1991
Stetter KO, Kandler O: Untersuchungen zur Entstehung vonDl-milchsäure bei lactobacillen und charakterisierung einer milchsäureracemase bei einigen Arten der Untergattung streptobacterium. Arch Mikrobiol 94:221–247, 1973
Bustos-Fernández L, González E, Ledesma de Paolo I, Celener D, Ogawa De Furuya K: Organic anions induce colonic secretion. Dig Dis 21:329–332, 1976
Fedorak RN, Empey LR, MacArthur C, Jewell LD: Misoprosol provides a colonic mucosal protective effect during acetic acid-induced colitis in rats. Gastroenterology 98:615–625, 1990
Saunders DR, Sillery BS: Effect of lactate and H+ on structure and function of rat intestine. Dig Dis Sci 27:33–41, 1982
Mortensen PB, Holtug K, Bonnén H, Clausen MR: The degradation of amino acids, proteins and blood to short-chain fatty acids in colon is prevented by lactulose. Gastroenterology 98:353–360, 1990
Mortensen PB, Holtug K, Rasmussen HS: Short-chain fatty acid production from mono- and disaccharides in a fecal incubation system: Implications for colonic fermentation of dietary fiber in humans. J Nutr 18:321–325, 1988
Rasmussen HS, Holtug K, Andersen JR, Krag E, Mortensen PB: The influence of ispagula husk and lactulose on thein vivo and thein vitro production capacity of short-chain fatty acids in humans. Scand J Gastroenterol 22:406–410, 1987
Rombeau JL, Kripke SA: Metabolic and intestinal effects of short-chain fatty acids. JPEN 14:181S-185S, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hove, H., Mortensen, P.B. Colonic lactate metabolism andd-lactic acidosis. Digest Dis Sci 40, 320–330 (1995). https://doi.org/10.1007/BF02065417
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02065417