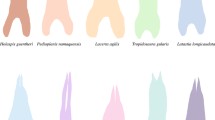
Abstract
Major squamate taxa exhibit extreme variation in lingual morphology, presumably due to correlated variation in trophic and chemosensory functions. Data are presented on evolution of lingual shape documenting several trends: (1) Resting lingual elongation is greatest in families specialized for lingual chemosensory sampling. (2) The greatest increase in elongation achievable by intralingual means including elasticity and foretongue retractility occurs in families with intermediate degrees of lingual specialization for chemosensory sampling. Sampling efficiency may be enhanced by the ability to extend the tongue well beyond the mouth, with resting elongation and intralingual extensibility perhaps jointly determining distance extended. In families lacking sufficient resting elongation, augmentation of intralingual extensibility may be a means of approaching optimal protrusion distances. Decreased extensibility evolved in tandem with the greatest resting elongation, suggesting that resting elongation may be more efficient for protrusion and that elasticity declines as optimal resting length is approached. The optimal shape for chemosensory sampling may be predicted to be highly elongate, as in teiids, varanids, and colubrids. The tongue should be broad at the tip for prehension (as in iguanians), fleshy for manipulation and swallowing, and broad at the base for tamping prey into the esophagus. (3) Lingual surface area relative to that of a rectangle of dimensions length × base width varies accordingly. Relative area is high in families that do not tongue-flick much while foraging because tongues are broad and fleshy throughout their length. It is low in families that have wedge-shaped tongues and intermediate specialization for chemosensory sampling. Narrowing of the anterior tongue may improve chemical sampling. Relative lingual area in chemosensory specialists is very high, with progressive narrowing toward the base as optimal sampling shape is approached in taxa lacking lingual function in swallowing, prehension or prey manipulation.
Similar content being viewed by others
References
Bels, V.L., Chardon, M., andKardong, K.V. 1994. Biomechanics of the hyolingual system in Squamata, pp. 197–240,in Advances in Comparative and Environmental Physiology, Vol. 18. Springer-Verlag, Berlin.
Camp, C. 1923. Classification of the lizards.Bull. Am. Mus. Nat. Hist. 48:289–481.
Cooper, W.E., Jr. 1989a. Absence of prey odor discrimination by iguanid and agamid lizards in applicator tests.Copeia 1989:472–478.
Cooper, W.E., Jr. 1989b. Prey odor discrimination in varanoid lizards: Responses of gila monsters (Heloderma suspectum) and savannah monitors (Varanus exanthematicus) to chemical stimuli presented on cotton swabs.Ethology 81:250–258.
Cooper, W.E., Jr. 1989c. Prey chemical discrimination by the broad-headed skink (Eumeces laticeps).J. Exp. Zool. 249:11–16.
Cooper, W.E., Jr. 1990a. Prey odor detection by teiid and lacertid lizards and the relationship of prey odor detection to foraging mode in lizard families.Copeia 1990:237–242.
Cooper, W.E., Jr. 1990b. Prey odor discrimination by anguid lizards.Herpetologica 46:183–190.
Cooper, W.E., Jr. 1991. Responses to prey chemicals by a lacertid lizard,Podarcis muralis: Prey chemical discrimination and poststrike elevation in tongue-flick rate.J. Chem. Ecol. 17:849–863.
Cooper, W.E., Jr. 1994a. Chemical discrimination by tongue-flicking in lizards: A review with hypotheses on its origin and its ecological and phylogenetic relationships.J. Chem. Ecol. 20:439–487.
Cooper, W.E., Jr. 1994b. Prey chemical discrimination, foraging mode, and phylogeny, pp. 95–116,in L.J. Vitt and E.R. Pianka (eds.). Lizard Ecology: Historical and Experimental Perspectives. Princeton University Press, Princeton.
Cooper, W.E., Jr. 1994c. Multiple functions of extraoral lingual behavior in iguanian lizards: Prey capture, grooming, and swallowing, but not prey detection.Anim. Behav. 47:765–775.
Cooper, W.E., Jr., andAlberts, A.C. 1990. Responses to chemical food stimuli by an herbivorous actively foraging lizard,Dipsosaurus dorsalis.Herpetologica 46:259–266.
Cooper, W.E., Jr., andBurghardt, G.M. 1990. Vomerolfaction and vomodor.J. Chem. Ecol. 16:103–105.
Estes, R., De Queiroz, K., andGauthier, J. 1988. Phylogenetic relationships within Squamata, pp. 119–281,in R. Estes and G. Pregill (eds.). Phylogenetic Relationships of the Lizard Families. Stanford University Press, Stanford, California.
Ford, N.B., andLow, J.R., Jr. 1983. Sex pheromone source location by snakes: A mechanism for detection of direction in nonvolatile trails.J. Chem. Ecol. 10:1193–1199.
Frost, D.R., andEtheridge, R. 1989. A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata).Misc. Publ. Univ. Kans. Mus. Nat. Hist. 81:1–65.
Gabe, M., andSaint Girons, H. 1976. Contribution a la morphologie comparee des fosses nasales et leurs annexes chez les lepidosauriens.Mem. Mus. Natl. Hist. Nat., Nouvelle Ser. A 98:1–87 + 49 figs., 10 pl.
Halpern, M. 1992. Nasal chemical senses in reptiles: structure and function, pp. 423–523,in C. Gans and D. Crews (eds.). Biology of the Reptilia, Vol. 18, Physiology and Behavior. University of Chicago Press, Chicago, Illinois.
Maddison, W.P., andMaddison, D.R. 1992. MacClade Analysis of Phylogeny and Character Evolution, Version 3.1. Sinauer Associates, Sunderland, Massachusetts.
Mason, R.T. 1992. Reptilian pheromones, pp. 114–228,in C. Gans and D. Crews (eds.). Biology of the Reptilia, Vol. 18, Physiology and Behavior. University of Chicago Press, Chicago, Illinois.
McDowell, S.B. 1972. The evolution of the tongue of snakes, and its bearing on snake origins, pp. 191–273,in T. Dobzhansky, M.K. Hecht, and W.C. Steere (eds.). Evolutionary Biology, Vol. 6. Appleton-Century-Crofts, New York, New York.
Schwenk, K. 1984. The Evolutionary Morphology of the Lepidosaur Tongue. PhD dissertation. University of California, Berkeley.
Schwenk, K. 1985. Occurrence, distribution, and functional significance of taste buds in lizards.Copeia 1985:91–101.
Schwenk, K. 1986. Morphology of the tongue in the tuatara,Sphenodon punctatus, with comments on function and phylogeny.J. Morphol. 188:129–156.
Schwenk, K. 1988. Comparative morphology of the lepidosaur tongue and its relevance to squamate phylogeny, pp. 569–598,in R. Estes and G. Pregill (eds.). Phylogenetic Relationships of the Lizard Families. Stanford University Press, Stanford, California.
Schwenk, K. 1993. The evolution of chemoreception in squamate reptiles: A phylogenetic approach.Brain Behav. Evol. 41:124–137.
Schwenk, K. 1994. Why snakes have forked tongues.Science 263:1573–1577.
Schwenk, K., andThrockmorton, G.S. 1989. Functional and evolutionary morphology of lingual feeding in squamate reptiles: Phylogenetics and kinematics.J. Zool. London 219:153–175.
Siegel, S. 1956. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill, New York.
Smith, K.K. 1984. The use of the tongue and hyoid apparatus during feeding in lizards (Ctenosaura similis andTupinambis nigropunctatus).J. Zool. London 202:115–143.
Sokal, R.R., andRohlf, F.J. 1981. Biometry, 2nd ed. W.H. Freeman, San Francisco, California.
Young, B.A. 1990. Is there a direct link between the ophidian tongue and Jacobson's organ?Amphibia-Reptilia 11:263–676.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cooper, W.E. Evolution and function of lingual shape in lizards, with emphasis on elongation, extensibility, and chemical sampling. J Chem Ecol 21, 477–505 (1995). https://doi.org/10.1007/BF02036744
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02036744