Abstract
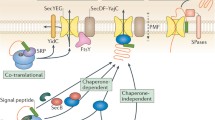
Proteins enter the secretory pathway by two general routes. In one, the complete polypeptide is made in the cytoplasm and held in an incompletely folded state by chaperoning adenosine triphosphatases (ATPases) such as hsp70. InSaccharomyces cerevisiae, fully synthesized secretory precursors engage the endoplasmic reticulum (ER) membrane by interaction with a set of Sec proteins comprising the polypeptide translocation apparatus (Sec61p, Sec62p, Sec63p, Sec71p, Sec72p). Productive interaction requires displacement of hsp70 from the precursor, a reaction that is facilitated by Ydj1p, a homologue of theEscherichia coli DnaJ protein. Both DnaJ and Ydj1p regulate chaperone activity by stimulating the ATPase activity of their respective hsp70 partners (E. coli DnaK andS. cerevisiae Ssa1p, resepectively). In the ER lumen, another hsp70 chaperone, BiP, binds ATP and interacts with the ER membrane via its contact with a peptide loop of Sec63p. This loop represents yet another DnaJ homologue in that it contains a region of ∼70 residue similarity to the ‘J box’, the most conserved region of the DnaJ family of proteins. In the presence of ATP, under conditions in which BiP can bind to Sec63p, the secretory precursor passes from the cytosol into the lumen through a membrane channel formed by Sec61 p. A second route to the membrane pore that is used by many other secretory precursors, particularly in mammalian cells, requires that the polypeptide engage the ER membrane as the nascent chain emerges from the ribosome. Such cotranslational translocation bypasses the need for certain Sec proteins, instead utilizing an alternate set of cytosolic and membrane factors that allows the nascent chain to be inserted directly into the Sec61p channel.
Similar content being viewed by others
References
von Heijne G. (1990) The signal peptide. J. Membr. Biol.115: 195–201
Verner K. and Schatz G. (1988) Protein translocation across membranes. Science241: 1307–1313
Gething M. and Sambrook J. (1992) Protein folding in the cell. Nature.355: 33–45
Rapoport T. A. (1992) Transport of proteins across the endoplasmic reticulum membrane. Science258: 931–936
Nunnari J. and Walter P. (1992) Protein targeting to and translocation across the membrane of the endoplasmic reticulum. Curr. Opin. Cell Biol.4: 573–580
Walter P. and Johnson A. E. (1994) Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Ann. Rev. Cell Biol.10: 87–119
Wiedmann B., Sakai H., Davis T. A. and Wiedmann M. (1994) A protein complex required for signal-sequence-specific sorting and translocation. Nature.370: 434–440
Powers T. and Walter P. (1995) Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPases. Science269: 1422–1423
Brown J. D., Hann B. C., Medzihradszky, K. F., Niwa, M., Burlingame A. L. and Walter P. (1994) Subunits of theSaccharomyces cerevisiae signal recognition particle required for its functional expression. EMBO J.13: 4390–4400
Wiech H., Sagstetter M., Muller G. and Zimmermann R. (1987) The ATP-requiring step in assembly of M13 procoat protein into microsomes is related to preservation of translocation competence of the precursor protein. EMBO J.6: 1011–1016
Zimmermann R., Sagstetter M., Lewis M. J. and Pelham H. R. B. (1988) Seventy-kilodalton heat shock proteins and an addition component from reticulocyte lysate stimulate import of M13 procoat into microsomes. EMBO J.7: 2875–2880
Takenaka I. M., Leung S., McAndrew S. J., Brown J. P. and Hightower L. E. (1995) Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J. Biol. Chem.270: 19839–19844
Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshultz R. J., Sprang S. R., Sambrook J. F. et al. (1993) Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell75: 717–728
Schmid D., Baici A., Gehring H. and Christen P. (1994) Kinetics of molecular chaperone action. Science263: 971–973
Palleros D. R., Shi L., Reid K. L. and Fink A. L. (1994) Hsp70-protein complexes: complex stability and conformation of bound substrate protein. J. Biol. Chem.269: 13107–13114
Hightower L. E., Sadis S. E. and Takenaka I. M. (1994) Interaction of vertebrate hsc70 and hsp70 with unfolded proteins and peptides. In: The Biology of Heat Shock Proteins and Molecular Chaperones, pp. 179–207. Morimoto R. I., Tissieres A. and Georgopoulos C. (eds), Cold Spring Harbor Laboratory Press, Plainview, NY
Craig E. A., Baxter B. K., Becker J., Halladay J. and Ziegelhoffer T. (1994) Cytosolic hsp70s ofSaccharomyces cerevisiae: roles in protein synthesis, protein translocation, proteolysis and regulation. In: The Biology of Heat Shock Proteins and Molecular Chaperones, pp. 31–52, Morimoto R. I., Tissieres A. and Georgopoulos C. (eds), Cold Spring Harbor Laboratory Press, Plainview, NY
Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A. and Schekman R. (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature332:800–805
Chirico W. J., Waters, M. G. and Blobel G. (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature332: 805–810
Cyr D. M., Langer T. and Douglas M. G. (1994) DnaJ-like proteins: molecular chaperones and specific regulators of hsp70. Trends Biochem. Sci.19: 176–181
Cyr D. M., Lu X. and Douglas M. G. (1992) Regulation of hsp70 function by a eukaryotic DnaJ homolog. J. Biol. Chem.267: 20927–20931
Caplan A. J., Cyr D. M. and Douglas M. G. (1992) YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell71: 1143–1155
Caplan A. J., Tsai J., Casey P. J. and Douglas M. G. (1992) Farnesylation of Ydj1p is required for function at elevated growth temperatures inSaccharomyces cerevisiae. J. Biol. Chem.267: 18890–18895
Langer T., Lu C., Echols H., Flanagan J., Hayer M. K. and Hartl F. U. (1992) Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature356: 683–689
Deshaies R. J., Sanders S. L., Feldheim D. A. and Schekman R. (1991) Assembly of the yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature349: 806–808
Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G. and Schekman R. (1989) Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J. Cell Biol.109: 2641–2652
Panzner S., Dreier L., Hartmann E., Kostka S. and Rapoport T. A. (1995) Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81: 561–570
Deshaies R. J. and Schekman R. (1990) Structural and functional dissection of Sec62p, a membrane-bound component of the yeast endoplasmic reticulum protein import machinery. Mol. Cell Biol.10: 6024–6035
Sanz P. and Meyer D. I. (1989) Secretion in yeast: preprotein binding to a membrane receptor and ATP-dependent translocation are sequential and separable events in vitro. J. Cell Biol.108: 2101–2106
Müsch A., Wiedmann M. and Rapoport T. A. (1992) Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell69: 343–352
Deshaies R. J. and Schekman R. W. (1989)SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J. Cell Biol.109: 2653–2664
Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D. and Schekman R. W. (1992) Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell69: 353–365
Green N., Fang H. and Walter P. (1992) Mutations in three novel complementation groups inhibit membrane protein insertion into and soluble protein translocation across the endoplasmic reticulum membrane ofSaccharomyces cerevisiae. J. Cell Biol.116: 597–604
Feldheim D., Yoshimura K., Admon A. and Schekman R. (1993) Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol. Biol. Cell4: 931–939
Feldheim D. and Schekman R. (1994) Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J. Cell Biol.126: 935–943
Fang H. and Green N. (1994) Nonlethalsec71-1 andsec72-1 mutations eliminate proteins associated with the Sec63-BiP complex fromS. cerevisiae. Mol. Biol. Cell5: 933–942
Brodsky J. L., Goeckeler J. and Schekman R. (1995) BiP and Sec63p are required for both co- and post-translational protein translocation into the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA92: 9643–9646
Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R. and Schekman R. (1992) Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol. Biol. Cell3: 129–142
Mothes W., Prehn S. and Rapoport T. A. (1994) Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J.13: 3973–3982
Görlich D., Prehn S., Hartmann E., Kalies K.-U. and Rapoport T. A. (1992) A mammalian homolog of Sec61p and SecYp is associated with ribosomes and nascent polypeptides during translocation. Cell71: 489–503
Kalies K.-U., Görlich D. and Rapoport T. A. (1994) Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J. Cell Biol.126: 925–934
Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J. and Silver P. (1989) A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, anEscherichia coli heat shock protein. J. Cell Biol.109: 2665–2675
Feldheim D., Rothblatt J. and Schekman R. (1992) Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol. Cell Biol.12: 3288–3296
Caplan A. J., Cyr D. M. and Douglas M. G. (1993) Eukaryotic homologues ofEscherichia coli DnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol. Biol. Cell4: 555–563
Vogel J. P., Misra L. M. and Rose M. D. (1990) Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol.110: 1885–1895
Nguyen T. H., Law D. T. S. and Williams D. B. (1991) Binding protein BiP is required for translocation of secretory proteins into the endoplasmic reticulum inSaccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA88: 1565–1569
Scidmore M. A., Okamura H. H. and Rose M. D. (1993) Genetic interactions betweenKAR2 andSEC63, encoding eukaryotic homologues of DnaK and DnaJ in the endoplasmic reticulum. Mol. Biol. Cell4: 1145–1159
Kaiser C. and Schekman R. (1990) Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell61: 723–733
Brodsky J. L. and Schekman R. (1993) A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J. Cell Biol.123: 1355–1363
Nelson M. K., Kurihara T. and Silver P. A. (1993) Extragenic suppressors of mutations in the cytoplasmic C terminus ofSEC63 define five genes inSaccharomyces cerevisiae. Genetics134: 159–173
Lyman S. K. and Schekman R. (1995) Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER ofSaccharomyces cerevisiae. J. Cell Biol.131: 1163–1171
Brodsky J. L. and Schekman R. (1994) Heat shock cognate proteins and polypeptide translocation across the endoplasmic reticulum membrane. In: The Biology of Heat Shock Proteins and Molecular Chaperones, pp. 85–109, Morimoto R. I., Tissieres A. and Georgopoulos C. (eds), Cold Spring Harbor Laboratory Press, Plainview, NY
Crowley K. S., Liao S., Worrell V. E., Reinhart G. D. and Johnson A. E. (1994) Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell78: 461–471
Glick B. S. (1995) Can hsp70 proteins act as force-generating motors? Cell80: 11–14
Brodsky J. L., Hamamoto S., Feldheim D. and Schekman R. (1993) Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and cytosolic hsc70. J. Cell Biol.120: 95–102
Ooi C. E. and Weiss J. (1992) Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell71: 87–96
Nicchitta C. V. and Blobel G. (1993) Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell73: 989–998
Görlich D. and Rapoport T. A. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell75: 615–630
Melnick J., Dul J. L. and Argon Y. (1994) Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature370: 373–375
Hammond C. and Helenius A. (1994) Folding of VSV G protein: sequential interaction with BiP and calnexin. Science266: 456–458
Ng D. T. W., Randall R. E. and Lamb R. A. (1989) Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuramidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J. Cell Biol.109: 3273–3289
Simons J. F., Ferro-Novick S., Rose M. D. and Helenius A. (1995) BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J. Cell Biol.130: 41–49
Schlenstedt G., Harris S., Risse B., Lill R. and Silver P. A. (1995) A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interaction with hsp70s. J. Cell Biol.129: 979–988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lyman, S.K., Schekman, R. Polypeptide translocation machinery of the yeast endoplasmic reticulum. Experientia 52, 1042–1049 (1996). https://doi.org/10.1007/BF01952100
Issue Date:
DOI: https://doi.org/10.1007/BF01952100