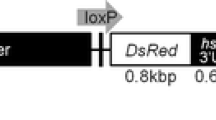
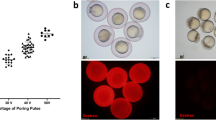
Abstract
Gene transfer into fish embryo is being performed in several species (trout, salmon, carps, tilapia, medaka, goldfish, zebrafish, loach, catfish, etc.). In most cases, pronuclei are not visible and microinjection must be done into the cytoplasm of early embryos. Several million copies of the gene are generally injected. In medaka, transgenesis was attempted by injection of the foreign gene into the nucleus of oocyte. Several reports indicate that the injected DNA was rapidly replicated in the early phase of embryo development, regardless of the origin and the sequence of the foreign DNA. The survival of the injected embryos was reasonably good and a large number reached maturity. The proportion of transgenic animals ranged from 1 to 50% or more, according to species and to experimentators. The reasons for this discrepancy have not been elucidated. In all species, the transgenic animals were mosaic. The copy number of the foreign DNA was different in the various tissues of an animal and a proportion lower than 50% of F1 offsprings received the gene from their parents. This suggests that the foreign DNA was integrated into the fish genome at the two cells stage or later. An examination of the integrated DNA in different cell types of an animal revealed that integration occurred mainly during early development. The transgene was found essentially unrearranged in the fish genome of the founders and offsprings. The transgenes were therefore stably transmitted to progeny in a Mendelian fashion. Southern blot analysis revealed the presence of possible junction fragments and also of minor bands which may result from a rearrangement of the injected DNA. In all species, the integrated DNA appeared mainly as random end-to-end concatemers. In adult trout blood cells, a small proportion of the foreign DNA was maintained in the form of non-integrated concatemers, as judged by the existence of end fragments. The transgenes were generally only poorly expressed. The majority of the injected gene constructs contained essentially mammalian or higher vertebrates sequences. The comparison of the expression efficiency of these constructs in transfected fish and mammalian cells indicates that some of the mammalian DNA sequences are most efficiently understood by the fish cell machinery. Chloramphenicol acetyl transferase gene under the control of promoters from Rous sarcoma virus, and human cytomegalovirus, was expressed in several tissues of transgenic fish. Chicken δ-crystallin gene was expressed in several tissues of transgenic fish. Rainbow trout growth hormone cDNA driven by the Rous sarcoma virus promoter was expressed in transgenic carps leading to a faster growth of these animals. The antifreeze protein gene from flounder was expressed in transgenic salmon. These data indicate that transgenesis in fish is relatively easy but that fish gene sequences must be preferably used to obtain a good expression of the transgenes. Fish is a good biological model, specially for developmental studies and it is an increasing part of human food. For these reasons, transgenesis in fish is most likely to be more and more practised in the coming years.
Similar content being viewed by others
References
Arezzo, F., Sea urchin sperm as vector of foreign genetic information. Cell. Biol. Int. Rep.13 (1989) 391–402.
Andres, A. C., Muellener, D. B., and Ryffel, G. O., Persistence, methylation and expression of vitellogenin gene derivatives after injection into fertilized eggs ofXenopus laevis. Nuc. Acids Res.12 (1984) 2283–2302.
Benyumov, A. O., Yenikopolov, G. N., Barmintsev, V. A., Zelenina, I. A., Sleptsova, L. A., Doronin, Y. K., Golichenkov, V. A., Graschuk, M. A., Georgiev, G. P., Rubtsov, P. M., Skryabin, G., and Baev, A. A., Integration and expression of human growth hormone gene in Teleostei. Genetika25 (1989) 24–35.
Blow, J. J., and Sleeman, A. M., Replication of purified DNA in Xenopus egg extract is dependent on nuclear assembly. J. Cell Sci.95 (1990) 383–391.
Brinster, R. L., Chen, H. Y., Trumbauer, M. E., Yagle, M. K., and Palmiter, R. D., Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. natl. Acad. Sci. USA82 (1985) 4438–4442.
Brem, G., Brenig, B., Hörstgen-Schwark, G., and Winnacker, E. L., Gene transfer in Tilapia (Oreochromis niloticus). Aquaculture68 (1988) 209–219.
Chen, T. T., Zhu, Z., Lin, C. M., Gonzalez-Villasenor, L. I., Dunham, R., and Powers, D. A., Fish genetic engineering: a novel approach in aquaculture, in: Proceeding National Shellfish Association Aquaculture 1989, Los Angeles, California, USA (in press).
Chong, S. S. C., and Vielkind, J. R., Expression and fate of CAT reporter gene microinjected into fertilized medaka (Oryzias latipes) eggs in the form of plasmid DNA, recombinant phage particles and its DNA. Theor. appl. Genet.78 (1989) 369–380.
Chourrout, D., Guyomard, R., and Houdebine, L. M., High efficiency gene transfer in rainbow trout (Salmo gairdneri Rich.) by microinjection into egg cytoplasm. Aquaculture5 (1986) 143–150.
Chourrout, D., Induction of gynogenesis, triploidy and tetraploidy in fish. ISI Atlas of Science; Animal Plant Sci. Sect.1 (1988) 65–70.
Chourrout, D., Guyomard, R., and Houdebine, L. M., Techniques for the development of transgenic fish: a review, in: Transgenic Models in Medecine and Agriculture, pp. 89–99. Ed. R. Church. Wiley-Liss, Inc., New York (1990).
Davies, P. L., Hew, C. L., Shears, M. A., and Fletcher, G. L., Antifreeze protein expression in transgenic salmon. J. cell. Biochem. Suppl.13 B (1989) 169.
Dreano, M., Marq, J. B., and Bromley, P., Antibody formation against heat-induced gene products expressed in animals. Biotechnology6 (1988) 1340–1342.
Dunham, R. A., Eash, J., Askins, J., and Townes, J. M., Transfer of metallothionein human growth hormone fusion gene into channel catfish. Trans. Am. Fish. Soc.116 (1987) 87–91.
Endean, D. J., and Smithies, O., Replication of plasmid DNA in fertilizedXenopus eggs in sensitive to both the topology and the size of the injected template. Chromosoma97 (1989) 307–316.
Etkin, L. D., Pearman, B., Roberts, M., and Bektesh, S. L., Replication, integration and expression of exogenous DNA injected into fertilized eggs ofXenopus laevis. Differentiation36 (1984) 194–202.
Etkin, L. D., and Pearman, B., Distribution, expression and germ line transmission of exogenous DNA sequences following microinjection intoXenopus laevis eggs. Development99 (1987) 15–23.
Fire, A., and Waterston, R. H., Proper expression of myosin genes in transgenic nematodes. EMBO J.8 (1989) 3419–2428.
Fletcher, G. L., Shears, M. A., King, M. J., Davies, P. L., and Hew, C. L., Evidence for antifreeze protein gene transfer in Atlantic salmon (Salmo salar). Can. J. Fish. aquat. Sci.45 (1988) 352–357.
Flytzanis, C. N., McMahon, A. P., Hough-Evans, B. R., Katula, K. S., Britten, R. J., and Davidson, E. H., Persistence and integration of cloned DNA in post embryonic sea urchin. Devl Biol.108 (1985) 431–442.
Forbes, D. J., Kirschner, N. W., and Newport, J. W., Spontaneous formation of nucleus-like structures around bacteriophage DNA microinjected intoXenopus eggs. Cell34 (1983) 13–23.
Freidereich, H., and Schartl, M., Transient expression directed by homologous and heterologous promoter and enhancer sequences in fish cells. Nucl. Acids Res.18 (1990) 3299–3305.
Gedamu, L., Olsson, P. E., Zafarullah, M., Control of metallothionein gene expression in the rainbow trout. J. cell. Biochem. Suppl.13 B (1989) 167.
Gilley, D., Preer, J. R., Aufderheide, K. J., and Polisky, B., Autonomous replication and addition of telomer-like sequences to DNA microinjected into Paramecium tetraurelia macronuclei. Molec. cell. Biol.8 (1988) 4765–4772.
Guyomard, R., Chourrout, D., Leroux, C., Houdebine, L. M., and Pourrain, F., Integration and germ line transmission of foreign genes microinjected into fertilized trout eggs. Biochimie71 (1989) 857–883.
Hayat, M., Joyce, C. P., Townes, T. M., Chen, T. T., Powers, D. A., and Dunham, R. A., Survival and integration rate of channel catfish and common carps embryos micro injected with DNA at various development stages. Aquaculture (in press).
Hough-Evans, B. R., Britten, R. J., and Davidson, E. H., Mosaic incorporation and regulated expression of an exogenous gene in sea urchin embryo. Devl Biol.129 (1988) 198–208.
Indig, E. E., and Moav, B., A procaryotic gene is expressed in fish cells and perists in tilapia embryos and adults following microinjection, in: Reproduction in Fish and Applied Aspects of Endocrinology and Genetics, pp. 221–225. INRA Press, Paris 1988.
Inoue, K., Ozato, K., Kondoh, H., Iwamatsu, T., Wakamatsu, Y., Fujita, T., and Okada, T. S., Stage-dependent expression of the chicken δ-crystallin gene in transgenic fish embryo. Cell Diff. Dev.27 (1989) 57–68.
Inoue, K., Yamashita, S., Hata, J. I., Kabeno, S., Asada, S., Nagahisa, E., and Fujita, T., Electroporation as a new technique for producing transgenic fish. Cell Diff. Dev.29 (1990) 123–128.
Kozlov, A. P., Reshetnikov, V. L., Korzh, V. P., and Neyfakh, A. A., The fate of plasmid DNA in the developing embryos of loach,Misgurnus fossilis L. Molec. Biol. (Russian)22 (1988) 1614–1622.
Lavitrano, M., Camaioni, A., Fazio, V. M., Dolci, S., Farace, M. G., and Spadafora, C., Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell57 (1989) 717–723.
Maclean, N., Penman, D., and Zhu, Z., Introduction of novel genes into fish. Biotechnology5 (1987) 257–281.
McMahon, A. P., Flytzanis, C. N., Hough-Evans, B. R., Katula, K. S., Britten, R. J., and Davidson, E. H., Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis germ. Devl Biol.108 (1985) 420–430.
McEvoy, T., Stack, M., Keane, B., Barry, T., Sreenan, J., and Gannon, F., The expression of foreign gene in salmon embryo. Aquaculture68 (1988) 27–37.
Marini, N. J., Etkin, L. D., and Benbow, B. M., Persistence and replication of plasmid DNA microinjected into early embryos ofXenopus laevis. Devl Biol.127 (1988) 421–434.
Michaeli, T., Dan, Z. Q., and Prives, C., An excised SV40 intron accumulates and is stableXenopus laevis oocytes. Genes Devt2 (1988) 1012–1020.
Niu, M. C., Xue, G. X., Niu, L. C., and Huang, H. Z., Transfer of information from mRNA to chromosomes by reverse transcription in early development of goldfish eggs. Cell. molec. Biol.35 (1989) 333–345.
Ozato, K., Kondoh, H., Inohara, H., Iwamatsu, T., Wakamatsu, Y., and Okada, T. S., Production of transgenic fish: introduction and expression of chicken δ-crystallin gene in medaka embryos. Cell. Diff.19 (1986) 237–244.
Pasleau, F., Leung, F., and Kopchick, J. J., A comparison of bovine growth hormone expression directed by bGH genomic or intronless DNA in transiently transfected eucaryotic cells. Gene57 (1987) 47–52.
Penman, D. J., Beeching, A. J., Penn, S., and Maclean, N., Factors affecting survival and integration following microinjection of novel DNA into rainbow trout egg. Aquaculture85 (1990) 35–50.
Powers, D. A., Fish as a model system. Science246 (1989) 352–358.
Robertson, E., Bradley, A., Kuehn, M., and Evans, M., Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature323 (1986) 445–448.
Rokkones, E., Allestrom, P., Skjervold, D. H., and Gautvik, K. M., Microinjection and expression of a mouse metallothionein human growth hormone gene in fertilized salmon eggs. J. comp. Physiol.B 158 (1989) 751–758.
Rubenstein, J. L. R., Nicolas, J. F., and Jacob, F., Introduction of genes into preimplantation mouse embryos by use of a defective recombinant retrovirus. Proc. natl Acad. Sci. USA83 (1986) 366–368.
Schneider, J. F., Hallerman, E. M., Yoon, S. J., He, L., Myster, S. A., Gross, M., Liu, Z., Zhu, Z., Hackett, P. B., Guise, K. S., Kapuscinski, A. R., and Faras, A. J., Microinjection and successful transfer of the bovine growth hormone gene into the Northern pikeEsox lucius. J. cell. Biochem. Suppl.13 B (1989) 173.
Spieth, J., MacMorris, M., Broverman, S., Greenspoon, S., and Blumenthal, T., Regulated expression of a vitellogenin fusion gene in transgenic nematodes. Devl Biol.130 (1988) 285–293.
Stinchcomb, D. T., Shaw, J. E., Carr, S. H., and Hirsh, D., Extrachromosomal DNA transformation of Caenorhabditis elegans. Molec. cell. Biol.5 (1985) 3484–3486.
Stuart, G. W., McMurray, J. V., and Westerfield, M., Replication, integration and germ-line transmission of foreign sequences injected into early zebrafish embryos. Development103 (1988) 405–442.
Stuart, G. W., McMurray, J. V., and Westerfield, M., Germ-line transformation of the zebrafish, in: Gene Transfer and Gene Therapy, pp. 19–28. Eds I. Verma, R. Mulligan and A. Beauset. Alan R. Liss Inc., New York 1989.
Stuart, G. W., Vielkind, J. R., McMurray, J. V., and Westerfield, M., Stable lines of transgenic zebra fish exhibit reproducible patterns of transgene expression. Development109 (1990) 577–584.
Tamiya, E., Sugiyama, T., Masaki, K., Hirose, A., Okoshi, T., and Karube, I., Spatial imaging of luciferase gene expression in transgenic fish. Nucl. Acids Res.18 (1990) 1072.
Thorgaard, G. H., Chromosome set manipulation and sex control in fish, in: Fish Physiology, vol. 9 B, pp. 405–434. Eds W. S. Hoar, D. J. Randall and E. M. Donaldson. Academic Press, New York 1983.
Yasui, W., and Ryoji, M., Characterization of early DNA synthesis in Xenopus eggs after injection of circular plasmid DNA Nucl. Acids Res.17 (1989) 3709–3723.
Yoon, S. J., Liu, Z., Kapuscinski, A. R., Hackett, P. B., Faras, A. J., and Guise, K. S., Successful gene transfer in fish, in: Gene Transfer and Gene Therapy, pp. 29–34. Eds I. Verma, R. Mulligan and A. Beauset. Alan R. Liss, Inc., New York 1989.
Yoon, S. J., Hallerman, E. M., Gross, M., Liu, Z., Schneider, J. F., Faras, A. J., Kapuscinski, A. R., and Guise, K. S., Transfer of the gene for neomycin resistance into goldfish.Carassius auratus. Aquaculture85 (1990) 21–33.
Yoshisaki, G., Oshiro, T., and Takashima, F., Prevention of hardening of chorion and dechorionation for microinjection into fish eggs. Nippon Suisan Gal kaishi55 (1989) 369.
Zhang, P., Hayat, M., Joyce, C., Gonzalez-Villasenor, L. I., Lin, C. M., Dunham, R. A., Chen, T. T., and Powers, D. A., Gene transfer expression and inheritance of pRSV-rainbow trout-GH. cDNA in the common carp,Cyprinus carpio (Linnaeus). Molec. Reprod. Dev.25 (1990) 3–13.
Zhu, Z., Li, G., He, L., and Chen, S., Novel gene transfer into the fertilized eggs of goldfish. Z. angew. Ichthyol.1 (1985) 31–34.
Zucchi, T. M. A. D., Passos, G. A. S., and De Lucca, F. L., RNA-mediated genetic transformation inAspergillus nidulans. Cell. molec. Biol.35 (1989) 573–580.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Houdebine, L.M., Chourrout, D. Transgenesis in fish. Experientia 47, 891–897 (1991). https://doi.org/10.1007/BF01929879
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01929879