Abstract
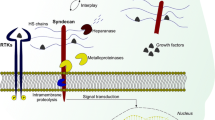
Tumor stroma is a specialized form of tissue that is associated with epithelial neoplasms. Recent evidence indicates that significant changes in proteoglycan content occur in the tumor stroma and that these alterations could support tumor progression and invasion as well as tumor growth. Our main hypothesis is that the generation of tumor stroma is under direct control of the neoplastic cells and that, via a feedback loop, altered proteoglycan gene expression would influence the behavior of tumor cells. In this review, we will focus primarily on the work from our laboratory related to the altered expression of chondroitin sulfate proteoglycan and its role in tumor development and progression. The connective tissue stroma of human colon cancer is enriched in chondroitin sulfate and the stromal cell elements, primarily colon fibroblasts and smooth muscle cells, are responsible for this biosynthetic increase. These changes can be reproduced in vitro by using either tumor metabolites or co-cultures of human colon carcinoma cells and colon mesenchymal cells. The levels of decorin, a leucine-rich proteoglycan involved in the regulation of matrix assembly and cell proliferation, are markedly elevated in the stroma of colon carcinoma. These changes correlate with a marked increase in decorin mRNA levels and a concurrent hypomethylation of decorin gene, a DNA alteration associated with enhanced gene expression. Elucidation of decorin gene structure has revealed an unexpected degree of complexity in the 5′ untranslated region of the gene with two leader exons that are alternatively spliced to the second coding exon. Furthermore, a transforming growth factor beta (TGF-β)-negative element is present in the promoter region of decorin gene. This regulatory domain is likely to be implicated in the silencing of decorin gene by TGF-β and may contribute to the regulation of this matrix gene in the tumor stroma.
Similar content being viewed by others
References
Adany, R., Heimer, R., Caterson, B., Sorrell, J. M., and Iozzo, R. V., Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. J. biol. Chem.265 (1990) 11389–11396.
Adany, R., and Iozzo, R. V., Altered methylation of versican proteoglycan gene in human colon carcinoma. Biochem. biophys. Res. Commun.171 (1990) 1402–1413.
Adany, R., and Iozzo, R. V., Hypomethylation of the decorin proteoglycan gene in human colon cancer. Biochem. J.276 (1991) 301–306.
Angel, P., Baumann, I., Stein, B., Delius, H., Rahmsdorf, H. J., and Herrlich, P., 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Molec. cell. Biol.7 (1987) 2256–2266.
Bennett, V. D., and Adams, S. L., Identification of a cartilagespecific promoter within intron 2 of the chick α2(I) collagen gene. J. biol. Chem.265 (1990) 2223–2230.
Bidanset, D. B., Guidry, C., Rosenberg, L. C., Choi, H. U., Timpl, R., and Hook, M., Binding of the proteoglycan decorin to collagon type VI. J. biol. Chem.267 (1992) 5250–5256.
Bird, A., The essentials of DNA methylation. Cell70 (1992) 5–8.
Bonaldo, P., Russo, V., Bucciotti, F., Doliana, R., and Colombatti, A., Structural and functional features of the α3 chain indicate a bridging role for the chicken collagen VI in connective tissues. Biochemistry29 (1990) 1245–1254.
Breibart, R. E., Andreadis, A., and Nadal-Ginard, B., Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. A. Rev. Biochem.56 (1987) 467–495.
Breuer, B., Schimdt, G., and Kresse, H., Non-uniform influence of transforming growth factor-β on the biosynthesis of different forms of small chondroitin sulphate/dermatan sulphate proteoglycan. Biochem. J.269 (1990) 551–554.
Broekelmann T. J., Limper, A. H., Colby, T. V., and McDonald, J. A., Transforming growth factorβ 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. natl Acad. Sci. USA88 (1991) 6642–6646.
Danielson, K. G., Fazzio, A., Cohen, I., Cannizzaro, L., Eichstetter, I., and Iozzo, R. V., Human decorin gene. Intronexon organization, discovery of two alternatively-spliced exons in the 5′ untranslated region and mapping of the gene to chromosome 12q23. Genomics15 (1993) 146–160.
Day, A. A., McQuillan, C. I., Termine, J. D., and Young, M. R., Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem. J.248 (1987) 801–805.
Dodge, G. R., Kovalszky, I., Chu, M-L., Hassell, J. R., McBride, O. W., Yi, H. F., and Iozzo, R. V., Heparan sulfate proteoglycan of human colon: Partial molecular cloning, cellular expression, and mapping of the gene (HSPG2) to the short arm of human chromosome 1. Genomics10 (1991) 673–680.
Dodge, G. R., Kovalszky, I., Hassell, J. R., and Iozzo, R. V., Transforming growth factor β alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J. biol. Chem.265 (1990) 18023–18029.
Esko, J. D., Genetic analysis of proteoglycan structure, function and metabolism. Curr. Opin. Cell Biol.3 (1991) 805–816.
Esko, J. D., Rostand, K. S., and Weinke, J. L., Tumor formation dependent on proteoglycan biosynthesis. Science241 (1988) 1092–1096.
Heino, J., Kähäri, V-M., Mauviel, A., and Krusius, T., Human recombinant interleukin-1 regulates cellular mRNA levels of dermatan sulfate proteoglycan core protein. Biochem. J.252 (1988) 309–312.
Iozzo, R. V., Neoplastic modulation of extracellular matrix: colon carcinoma cells release polypeptides that alter proteoglycan metabolism in colon fibroblasts. J. biol. Chem.260 (1985) 7464–7473.
Iozzo, R. V., Proteoglycans and neoplasia. Cancer Metastasis Rev.7 (1988a) 39–50.
Iozzo, R. V., Cell surface heparan sulfate proteoglycan and the neoplastic phenotype. J. cell. Biochem.37 (1988b) 61–78.
Iozzo, R. V., Bolender, R. P., and Wight, T. N., Proteoglycan changes in the intercellular matrix of human colon carcinoma. Lab. Invest.47 (1982) 124–138.
Iozzo, R. V., and Müller-Glauser, W., Neoplastic modulation of extracellular matrix: Proteoglycan changes in the rabbit mesentery induced by V2 carcinoma cells. Cancer Res.45 (1985) 5677–5687.
Iozzo, R. V., Sampson, P. M., and Schmitt, G., Neoplastic modulation of extracellular matrix: stimulation of chondroitin sulfate proteoglycan and hyaluronic acid synthesis in co-cultures of human colon carcinoma and smooth muscle cells. J. cell. Biochem.39 (1989) 355–378.
Iozzo, R. V., and Wight, T. N., Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J. biol. Chem.257 (1982) 11135–11144.
Kähäri, V-M., Larjava, H., and Uitto, J., Differential regulation of extracellular matrix proteoglycan gene expression. J. biol. Chem.266 (1991) 10608–10615.
Kähäri, V-M., Olsen, D. R., Rhudy, R. B., Carrillo, P., Chen Y. Q., and Uitto, J., Transforming growth factor-β upregulates elastin gene expression in human skin fibroblasts. Lab. Invest.66 (1992) 580–588.
Kerr, L. D., Miller, D. B., and Matrisian, L. M., TGF-β1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell61 (1990) 267–278.
Kjellén, L., and Lindhal, U., Proteoglycans: structures and interactions. A. Rev. Biochem.60 (1991) 443–475.
Knudson, W., Biswas, C., and Toole, B. P., Interaction between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc. natl Acad. Sci. USA81 (1984) 6767–6771.
Kokenyesi, R. and Woessner, F. Jr., Purification and characterization of a small dermatan sulphate proteoglycan implicated in the dilatation of the rat uterine cervix. Biochem. J.260 (1989) 413–419.
Krusius, T., and Ruoslahti, E., Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc. natl Acad. Sci. USA83 (1986) 7683–7687.
Li, W., Vergnes, J. P., Cournet, P. K., and Hassell, J. R., cDNA clone to chick corneal chondroitin/dermatan sulfate proteoglycan reveals identity to decorin. Archs Biochem. Biophys.296 (1992) 190–197.
Merrilees, M. J., and Finlay, G. J., Human tumor cells in culture stimulate glycosaminoglycan synthesis by human skin fibroblasts. Lab. Invest.53 (1985) 30–36.
Moses, H. L., Yang, E. Y., and Pietenpol, J. A., TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell63 (1990) 245–247.
Murdoch A. D., Dodge, G. R., Cohen, I., Tuan, R. S., and Iozzo, R. V., Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/Perlecan): A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules and epidermal growth factor. J. biol. Chem.267 (1992) 8544–8557.
Nathan, C., and Sporn, M., Cytokines in context. J. Cell Biol.113 (1991) 981–986.
Nishimura, I., Muragaki, Y., and Olsen, B. R., Tissue-specific forms of type IX collagen-proteoglycan arise from the use of two widely separate promoters. J. biol. Chem.264 (1989) 20033–20041.
Ritzenthaler, J. D., Goldstein, R. H., Fine, A., Lichtler, A., Rowe, D. W., and Smith, B. D., Transforming-growth-factor-β activation elements in the distal promoter regions of the rat α1 type I collagen gene. Biochem. J.280 (1991) 157–162.
Romaris, M., Heredia, A., Molist, A., and Bassols, A., Differential effect of transforming growth factor β on proteoglycan synthesis in human embryonic lung fibroblasts. Biochim. biophys. Acta1093 (1991) 229–233.
Ruoslahti, E., Structure and biology of proteoglycans. A. Rev. Cell Biol.4 (1988) 229–255.
Ruoslahti, E., and Yamaguchi, Y., Proteoglycans as modulators of growth factor activities. Cell64 (1991) 867–869.
Saitta, B., Timpl, R., and Chu, M-L., Human α2(VI) collagen gene. Heterogeneity at the 5′ untranslated region generated by an alternate exon. J. biol. Chem.267 (1992) 6188–6196.
Saksela, O., Moscatelli, D., Sommer, A., and Rifkin, D. B., Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol.107 (1988) 743–751.
Schmidt, G., Robenek, H., Harrach, B., Glössl, I., Nolte, V., Hörmann, H., Richter, H., and Kresse, H., Interaction of the small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J. Cell Biol.104 (1987) 1683–1691.
Scott, J. E., and Orford, C. R., Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem. J.197 (1981) 213–216.
Shaw, G., and Kamen, R., A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation Cell46 (1986) 659–667.
Simionescu, D., Iozzo, R. V., and Kefalides, N. A., Bovine pericardial proteoglycan: Biochemical, immunochemical and ultrastructural studies. Matrix9 (1989) 301–310.
Stevenson, B. J., Hagenbuechle, O., and Wellauer, P. K., Sequence organization and transcriptional regulation of the mouse elastase II and trypsin gene. Nucl. Acids Res.14 (1986) 8307–8330.
Van den Hoff, A., Stromal involvement in malignant growth. Adv. Cancer Res.50 (1988) 158–196.
Vigny, M., Ollier-Hartmann, M. P., Lavigne, M., Fayein, N., Jeanny, J. C., Laurent, M., and Courtois, Y. Specific binding of basic fibroblast growth factor to basement membrane-like structures and to purified heparan sulfate proteoglycan of the EHS tumor. J. cell. Physiol.137 (1989) 321–328.
Vogel, K. G., Paulsson, B. M., and Heinegård, D., Specific inhibition of type I and II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J.223 (1984) 587–597.
Yamaguchi, Y., and Rouslahti, E., Expression of human proteoglycan in Chinese ovary cells inhibits cell proliferation. Nature336 (1988) 244–246.
Yamaguchi, Y., Mann, D. M., and Ruoslahti, E., Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature346 (1990) 281–284.
Yeo, T-K., Brown, L., and Dvorak, H. F., Alterations in proteoglycan synthesis common to healing wounds and tumors. Am. J. Path.138 (1991) 1437–1450.
Zimmermann, D. R., and Ruoslahti, E., Multiple domains of the large fibroblast proteoglycan, versican. EMBO J.8 (1989) 2975–2981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iozzo, R.V., Cohen, I. Altered proteoglycan gene expression and the tumor stroma. Experientia 49, 447–455 (1993). https://doi.org/10.1007/BF01923588
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01923588