Abstract
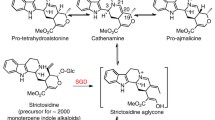
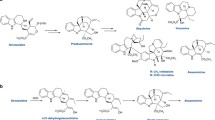
The harmonization of biosynthetic pathways with organic reaction mechanisms has relied heavily on stereochemical studies. The field of biosynthesis of pyrrolizidine alkaloids exemplifies these connections through a wide range of common organic reactions including oxidation, condensation, and decarboxylation. Further, the applications of biogenetic concepts and enzyme-catalysed reactions to synthesis are illustrated. The results are exciting, not only for their intrinsic scientific interest, but because they point the way to using plant enzymes to recognise structurally modified biosynthetic intermediates and hence open routes to the synthesis of new compounds that would otherwise be difficult to obtain.
Similar content being viewed by others
References
Battersby, A. R., Staunton, J., and Summers, M. C., Studies of enzyme-mediated reactions. Part VI. Stereochemical course of the dehydrogenation of stereospecifically labelled benzylamines by the amine oxidase from pea seedlings. J. Chem. Soc., Perkin Trans.1 (1976) 1052–1055.
Bentley, R., Molecular Asymmetry in Biology, vol. 2, p. 18. Academic Press, New York 1970.
Grue-Sorensen, G., and Spenser, I. D., Biosynthesis of retronecine. J. Am. chem. Soc.103 (1981) 3208–3210.
Grue-Sorensen, G., and Spenser, I. D., Biosynthesis of retronecine. Can. J. Chem.60 (1982) 643–662.
Grue-Sorensen, G., and Spenser, I. D., Deuterium nuclear magnetic resonance spectroscopy as a probe of the stereochemistry of biosynthetic reactions: the biosynthesis of retronecine. J. Am. chem. Soc.105 (1983) 7401–7404.
Kelly, H. A., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Incorporation of13C-labelled precursors into rosmarinine. J. Chem. Soc., Perkin Trans.1 (1987) 177–180.
Kelly, H. A., and Robins, D. J., Pyrrolizidine alkaloids. Stereochemistry of the enzymic processes involved in the biosynthesis of rosmarinecine. J. Chem. Soc., Perkin Trans.1 (1987) 2195–2202.
Kelly, H. A., and Robins, D. J., Evidence for an immonium ion intermediate in pyrrolizidine alkaloid biosynthesis. J. chem. Soc., chem. Commun. (1988) 329–330.
Khan, H. A., and Robins, D. J. Pyrrolizidine alkaloid biosynthesis; Incorporation of13C-labelled precursors into retronecine. J. chem. Soc., chem. Commun. (1981) 146–147.
Khan, H. A., and Robins, D. J., Pyrrolizidine alkaloids: Evidence forN-(4-aminobutyl)-1,4-diaminobutane (homospermidine) as an intermediate in retronecine biosynthesis. J. chem. Soc., chem. Commun. (1981) 554–556.
Khan, H. A., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Synthesis of13C-labelled putrescines and their incorporation into retronecine. J. chem. Soc., Perkin Trans.1 (1985) 101–105.
Khan, H. A., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Synthesis of14C-labelled homospermidines and their incorporation into retronecine. J. chem. Soc., Perkin Trans.1 (1985) 819–824.
Kunec, E. K., and Robins, D. J., Stereochemistry of pyrrolizidine alkaloid biosynthesis: Incorporation of chiral [2-2H]putrescines into retrorsine. J. chem. Soc., chem. Commun. (1985) 1450–1452.
Kunec, E. K., and Robins, D. J., Evidence for different 1-hydroxymethylpyrrolizidines as intermediates in the biosynthesis of retronecine and rosmarinecine. J. chem. Soc., chem. Commun. (1986) 250–252.
Kunec, E. K., and Robins, D. J., Application of2H NMR spectroscopy to study the incorporation of enantiomeric [2-2H]labelled putrescines into the pyrrolizidine alkaloid retrorsine. J. chem. Soc., Perkin Trans.1 (1987) 1089–1093.
Kunec, E. K., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Synthesis of3H-labelled trachelanthamidine and isoretronecanol and their incorporation into two pyrrolizidine bases (necines). J. chem. Soc., Perkin Trans.1 (1989) 1437–1441.
Leete, E., and Rana, J., Synthesis of [3,5-14C]trachelanthamidine and [1-3H]isoretronecanol and their incorporation into the retronecine moiety of riddelliine inSenecio riddellii J. nat. Prod.49 (1986) 838–844.
Mattocks, A. R., in: Chemistry and Toxicology of Pyrrolizidine Alkaloids. Academic Press, New York 1986.
Orr, G. R., and Gould, S. J., Stereochemistry of the bacterial ornithine, lysine, and arginine decarboxylase reactions. Tetrahedron Lett.23 (1982) 3139–3142.
Rana, J., and Leete, E., Biosynthesis of riddelliine: Incorporation of [3,5-14C]trachelanthamidine and [5-3H]isoretronecanol into the retronecine moiety of the alkaloid. J. chem. Soc., chem. Commun. (1985) 1742–1743.
Rana, J., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Intact incorporation of [1,9-13C2]homospermidine into retronecine, J. chem. Res. (S) (1983) 146–147.
Rana, J., and Robins, D. J., Pyrrolizidine alkaloid biosynthesis; Incorporation of2H-labelled putrescines into retronecine. J. chem. Soc., chem. Commun. (1983) 1222–1224.
Rana, J., and Robins, D. J., Stereochemistry of pyrrolizidine alkaloid biosynthesis: Incorporation of chiral [1-2H]putrescines into retrorsine. J. chem. Soc., chem. Commun. (1984) 517–519.
Rana, J., and Robins, D. J., Application of2H NMR spectroscopy to study the incorporation of2H-labelled putrescines into the pyrrolizidine alkaloid retrorsine. J. chem. Soc., Perkin Trans.1 (1986) 983–988.
Richards, J. C., and Spenser, I. D., The stereochemistry of the enzymic decarboxylation of L-arginine and L-ornithine. Can. J. Chem.60 (1982) 2810–2820.
Robins, D. J., The pyrrolizidine alkaloids, in: Progress in the Chemistry of Organic Natural Products, vol. 41, pp. 115–203. Eds W. Herz, H. Grisebach and G. W. Kirby. Springer-Verlag, Vienna/New York 1982.
Robins, D. J., A biogenetically patterned synthesis of the pyrrolizidine alkaloid, trachelanthamidine. J. chem. Soc., chem. Commun. (1982) 1289–1290.
Robins, D. J., Pyrrolizidine alkaloid biosynthesis. Synthesis of [1,2-13C2]putrescine and its incorporation into retronecine. J. chem. Res. (S) (1983) 326–327.
Robins, D. J., Stereochemistry of reactions catalysed by arginine decarboxylase and ornithine decarboxylase. Phytochemistry22 (1983) 1133–1135.
Robins, D. J., Pyrrolizidine alkaloids. Nat. Prod. Rep.1 (1984) 235–243;2 (1985) 213–220;3 (1986) 297–305;4 (1987) 577–590;6 (1989) 221–230;7 (1990) 377–386;8 (1991) 213–221.
Robins, D. J., Biosynthesis of pyrrolizidine alkaloids. Chem. Soc. Rev.18 (1989) 375–408.
Robins, D. J., and Sweeney, J. R., Pyrrolizidine alkaloids. Evidence for the involvement of spermidine and spermine in the biosynthesis of retronecine. J. chem. Soc., chem. Commun. (1979) 120–121.
Robins, D. J., and Sweeney, J. R., Pyrrolizidine alkaloid biosynthesis. Incorporation of14C-labelled precursors into retronecine. J. chem. Soc., Perkin Trans.1 (1981) 3083–3086.
Robins, D. J., and Sweeney, J. R., Pyrrolizidine alkaloid biosynthesis. Derivation of retronecine from L-arginine and L-ornithine. Phytochemistry22 (1983) 457–459.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robins, D.J. Stereochemistry of enzymic processes in the biosynthesis of pyrrolizidine alkaloids. Experientia 47, 1118–1122 (1991). https://doi.org/10.1007/BF01918375
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01918375