Abstract
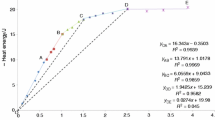
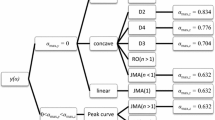
The kinetics of the reaction of one-to-one molar mixtures of crystalline silica and carbon powder were studied using thermogravimetric analysis. The resulting kinetic data was evaluated using simple kinetic and mass transport models. A two-stage reaction mechanism consisting of three stoichiometric reactions can adequately be used to describe the global reaction phenomena. Both the first and second stages of reaction were found to be influenced by diffusion mass transfer within the reacting bed of solids.
Zusammenfassung
Mittels Thermogravimetrie wurde die Reaktionskinetik der Reaktion von kristallinem Siliziumdioxid und Kohlenstoffpulver im Molverhältnis 1∶1 untersucht. Die kinetischen Angaben wurden mittels einfachen kinetischen und Stofftransportmodellen ausgewertet. Zu einer adäquaten Beschreibung der gesamten Reaktionserscheinung kann ein Zweischrittereaktionsmechanismus bestehend aus drei stöchiometrischen Reaktionen benutzt werden. Sowohl der erste als auch der zweite Reaktionsschritt wird durch diffusiven Stofftransport innerhalb des Reaktionsbettes beeinflußt.
Резюме
С помощью термограви метрического анализ а изучена кинетика реа кции кристаллической дву окиси кремния и порош кообразного углерода, взятые в мол ярном соотношении 1∶1. Резуль таты данных кинетики были оценены с помощью про стой кинетической модели и масс-перенос а. Двухстадийный реак ционный механизм, состоящий и з трех стехиометрических р еакций, может быть аде кватно использован для опис ания глобального реакционного явлени я. Установлено, что обе стадии реакции затрагивают ся диффузионным массопереносом в пре делах реакционного с лоя твердых тел.
Similar content being viewed by others
References
J. J. Biernacki, “Formation of Silicon Monoxide and Application to the Growth of Vapor-Liquid-Solid Silicon Carbide Whiskers”, Doctoral Dissertation, Fenn College of Engineering, Cleveland State University, Cleveland, Ohio, 1987, p. 378.
N. Klinger, E. L. Strauss and K. L. Kosmarek, J. Amer. Cer. Soc., 49 (1966) 369.
J.-G. Lee and I. B. Cutler, Cer. Bul., 65 (1975) 195.
F. Viscomi and L. Himmel, J. Metals, (1978) 21.
J. B. Henderson and M. R. Tant, Polymer Composites, 4 (1983) 233.
Fisher Scientific S-153 240 mesh floated silica (α-quartz).
R. T. Vanderbilt Company, Inc., Termax carbon powder.
H. E. Kissinger, J. Res. Nat. Bureau Stand., 57 (1956) 217.
E. S. Freeman and B. Carroll, J. Phys. Chem., 62 (1957) 394.
A. W. Coats and J. P. Redfern, Nature, 201 (1964) 68.
H. L. Friedman, J. Pol. Sci. C, 6 (1965) 183.
T. Ozawa, Bull. Chem. Soc. Japan, 38 (1965) 1881.
J. H. Sharp and S. A. Wentworth, Anal. Chem., 41 (1969) 2060.
J. H. Flynn, Organ. Plastics Chem., 44 (1981) 657.
J. P. Elder, J. Thermal Anal., 30 (1985) 657.
Details of this calculation are summarized in appendix VIII of reference 1.
Author information
Authors and Affiliations
Additional information
This paper is based on the doctoral dissertation of the senior author.
Rights and permissions
About this article
Cite this article
Biernacki, J.J., Wotzak, G.P. Thermogravimetric study of the C + SiO2 reaction. Journal of Thermal Analysis 35, 1651–1667 (1989). https://doi.org/10.1007/BF01912940
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01912940