Abstract
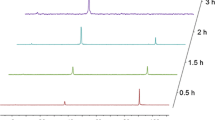
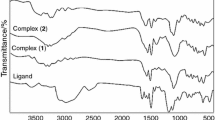
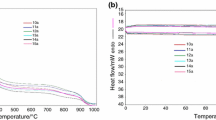
A new series of complexes of Zn(II), Cd(II) and Hg(II) with phosphoramidothioate (PAT), phosphorodiamidothioate (PDAT) and N,N′-diphenylphosphorodiamidothioate (N,N′-DPDAT) ligands have been synthesized and studied. The structures of the new compounds are discussed on the basis of their spectroscopic and thermal data. Thermoanalytical studies of these compounds have shown a dependence of the thermal stability on the size of the metal cation; the PAT complexes have greater thermal stability than the others.
Zusammenfassung
Komplexe von Zn(II), Cd(II) und Hg(II) mit Phosphoramidothioat (PAT), Phosphorodiamidothioat (PDAT) und N,N′-Diphenylphosphorodiamidothioat (N,N′-DPDAT) als Liganden wurden synthetisiert und untersucht. Die Strukturen der neuen Verbindungen werden basierend auf deren spektroskopischen und thermischen Daten diskutiert. Die thermoanalytische Untersuchung dieser Verbindungen hat eine Abhängigkeit der thermischen Stabilität von der Größe der Metallkationen ergeben, und zwar sind die PAT-Komplexe thermisch stabiler als die anderen.
Резюме
Синтезирована и изуч ена новая серия комплексов двухвале нтных цинка, кадмия и ртути с такими лиганд ами как фосфорамидот иокислота, фосфордиамидотиоки слота и N,N′дифенилфосф ордиамидотиокислот а. N,N′дифенилфосфордиам идотиокислота. Структура новых соед инений обсуждена на о снове ИК спектроскопических и термических данных. Термические исследования этих со единений показали зависимост ь их термоустойчивос ти от размера катиона. Комплексы с ф осфорамидотиокисло той обладали наибольшей устойчивостью.
Similar content being viewed by others
References
W. Antenrieth and P. Rudolph, Ber., 2 (1900) 2099.
H. Falius, Chem. Ber., 98 (1965) 3270.
H. Behrens and L. Huber, Chem. Ber., 93 (1960) 1137.
A. Syngollitou-Kourakou, I. Tossidis and M. Sigalas, Inorg. Chim. Acta, in press.
Watanabe Makoto, Inagaki Toshihiko and Sato Shoji, Bull. Chem. Soc. Jpn., 56 (1983) 458.
J. Dickert and C. Rowe, J. Org. Chem., 32 (1967) 647.
E. Merck, “Complexometric Assay Methods with Titriplex”, Darmstadt, 3rd edit. p. 24, 45, 55.
E. Steger and J. Frei, Z. Anorg. Allgem. Chem., 342 (1966) 195.
J. Frei and E. Steger, Z. Anorg. Allgem. Chem., 344 (1966) 194.
G. Sartri, C. Furlani and A. Damiani, J. Inorg. Nucl. Chem., 8 (1959) 119.
J. Fujita, K. Nakamoto and M. Kobayashi, J. Amer. Chem. Soc., 78 (1956) 3295.
M. Goodgame and A. Hayao, J. Chem. Soc., (A) (1968) 1108.
D. Adams, “Metal-Ligand and Related Vibrations”. Edward Arnold, London, 1967.
S. Lawton and G. Kokatailo, Inorg. Chem., 8 (1969) 2410.
S. Lawton, Inorg. Chem., 10 (1971) 328.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tossidis, I., Syngollitou-Kourakou, A. Structural and thermal analysis of phosphoramidothioate, phosphorodiamidothioate and N,N′-diphenylphosphorodiamidothioate complexes of Zn(II), Cd(II) and Hg(II). Journal of Thermal Analysis 32, 491–503 (1987). https://doi.org/10.1007/BF01912701
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01912701