Summary
Left ventricular collagen of various mammals (cat, cow, dog, pig, and rat) was successively extracted with neutral salt, and dilute acid solutions, and limited pepsin digestion. The distribution of the various types of collagen molecules in pepsin-solubilized ventricular collagen was analyzed electrophoretically on SDS-polyacrylamide gels in the presence of 3.6 M urea.
Yields of dilute-acid-soluble collagen were only 0.4 to 0.6% of the total ventricular collagen, and less than 0.1% with neutral salt solution. Approx. 55–90% of the total collagen was extracted by limited pepsin digestion.
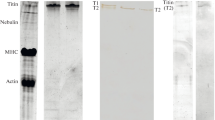
Disc patterns of pepsin-soluble collagen revealed the presence of dimeric and trimeric components, as well as higher-molecular-weight aggregates in all samples of nonreduced and reduced ventricular collagen. Taken together, these findings suggest the presence of an extensive interchain and intermolecular cross-linking network in left ventricular collagen.
Comparison of electrophoretical disc gel patterns of reduced and nonreduced pepsin-solubilized collagen indicated that left ventricular collagen is heterogenous in nature, consisting of a mixture of type I and type III collagen. It was evident that primarily type I components occur in ventricular collagen. The components of type III collagen molecules occurred in all investigated left ventricles in varying and consistently appreciably lower amounts. The proportions of type III and type I collagen vary in left ventricular tissue of different species.
Zusammenfassung
Kollagen aus dem linken Ventrikel verschiedener Säugetiere (Katze, Kuh, Hund, Schwein und Ratte) wurde sukzessiv mit neutraler Salz- und verdünnten Säurelösungen sowie mit limitierter Pepsinverdauung extrahiert.
Die Verteilung verschiedener Kollagentypen in pepsingelöstem Kollagen wurde mit Hilfe der SDS-Plyacrylamid-Gel-Elektrophorese analysiert.
Mit Salz- und Säurelösungen ließ sich weniger als 1% des Gesamtkollagens extrahieren. Begrenzte Pepsinverdauung löste 55–90% des Gesamtkollagens.
Von allen Proben des nichtreduzierten und reduzierten Kollagens wurden in Elektrophorese-Gelen di- und trimere Komponenten sowie auch hochmolekulare Aggregate von Kollagenmolekülen sichtbar. Diese Befunde weisen auf eine umfangreiche intra- und intermolekulare Vernetzung der Kollagenmoleküle im linken Ventrikel der Säugetiere hin.
Der Vergleich von Proteinbanden in Elektrophorese-Gelen von nichtreduziertem und reduziertem Kollagen zeigt, daß Kollagen vom linken Ventrikel heterogen ist, bestehend aus einem Gemisch von Kollagentyp I und-typ III. Vorwiegend ist Kollagentyp I vertreten. Komponenten vom Typ III kommen in allen untersuchten Proben vor, jedoch in unterschiedlichen und erheblich kleineren Proportionen.
Similar content being viewed by others
References
Gay, S., E. J. Miller: In: “Collagen in the Physiology and Pathology of Connective Tissue” (Stuttgart-New York, 1978).
Miller, E. J.: Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol. Cell. Biochem.130, 165–192 (1975).
Wiley, R. E., E. P. McClain: Isolation and characterization of Type III collagen from bovine cardiac muscle. Int. J. Biochem.,9, 139–143 (1978).
Epstein, E. H., N. H. Munderloh: Isolation and characterisation of CNBr peptides of human /α1 (III)/3 collagen and tissue distribution of /α1 (I)/2 α2 and α1 (III)/3 collagens. J. Biol. Chem.250, 9304–9312 (1975).
Medugorac, I.: Myocardial collagen in different forms of heart hypertrophy in the rat. Res. Exp. Med. (Berl.)177, 201–211 (1980).
McClain, P. E.: Characterisation of cardiac muscle collagen (molecular heterogeneity). J. Biol. Chem.249, 2303–2311 (1974).
Neumann, R. E., M. A. Logan: The determination of hydroxyproline. J. Biol. Chem.184, 299–306 (1950).
Hayashi, T., Y. Nagai: Separation of the α chains of type I and III collagens by SDS-polyacrylamide gel electrophoresis. J. Biochem.86, 453–459 (1979).
Chung, E., J. E. Miller: Collagen polymorphism: Characterisation of molecules with chain composition /α1 (III)/3 in human tissues. Science183, 1200–1201 (1974).
McClain, E. P., C. S. Morris: Cross-linking characteristics of cardiac muscle collagen. Proc. Soc. Exp. Biol. Med.143, 1127–1130 (1973).
McClain, P. E., J. G. Creed, R. E. Wiley J. R. Gerrits: Cross-linking characteristics of collagen from porcine intramuscular connective tissue: variation between muscles. Biochim. biophys. Acta221, 349–356 (1970).
Epstein jr., E. H. /α1 (III)/3 human skin collagen. J. Biol. Chem.249, 3225–3231 (1974).
Bailey, A. J., T. J. Sims: Chemistry of the collagen cross-links (nature of the cross-links in the polymorphic forms of dermal collagen during development). Biochem. J.153, 211–215 (1975).
Seyer, J. M., T. Eldridge, T. E. Hutcheson, A. H. Kang: Collagen polymorphism in normal liver and cirrhotic human liver. J. Clin. Invest.59, 241–248 (1977).
Seyer, J. M., T. E. Hutcheson, H. A. Kang; Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J. Clin. Invest. 1498–1507 (1976).
Gay, S., L. Weber, W. N. Meigel: Skin diseases: identification bei immunofluorescence of different collagen types. Immunol. Meeting Amsterdam 1975.
Narayanan, A. S., R. C. Page: Biochemical characterisation of collagen synthetized by fibroblasts derived from normal and diseased human gingiva. J. Biol. Chem.251, 5464–5471 (1976).
Medugorac, I.: Collagen content in different areas of normal and hypertrophied rat myocardium. Cardiovasc. Res.14, 551–554 (1980).
Author information
Authors and Affiliations
Additional information
Supported by grants from the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Medugorac, I. Characterization of intramuscular collagen in the mammalian left ventricle. Basic Res Cardiol 77, 589–598 (1982). https://doi.org/10.1007/BF01908312
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01908312