Abstract
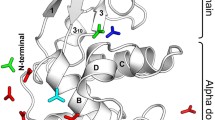
Similarities in amino acid sequences, three-dimensional structures, and the exon-intron patterns of their genes have indicated thatc-type lysozymes andα-lactalbumins are homologous proteins, i.e., descended by divergent evolution from a common ancestor. Like theα-lactalbumins, echidna milk, horse milk, and pigeon eggwhite lysozymes all bind Ca(II). Models of their three-dimensional structures, based on their amino acid sequences and the known crystal structures of domestic hen eggwhite and human lysozymes and baboon and humanα-lactalbumins, have been built. The several structures have been compared and their relationships discussed.
Similar content being viewed by others
References
Acharya, K. R., Stuart, D. L., Walker, N. P. C., Lewis, M., and Phillips, D. C. (1989).J. Mol. Biol. 208, 99–127.
Acharya, K. R., Stuart, D. I., Phillips, D. C., and Scheraga, H. A. (1990).J. Protein Chem. 9, 549–563.
Acharya, K. R., Ren, J., Stuart, D. I., Phillips, D. C., and Fenna, R. E. (1991).J. Mol. Biol. 221, 571–581.
Aqvist, J., van Gunsteren, W. F., Leijonmarck, M., and Tapia, O. (1985).J. Mol. Biol. 183, 461–477.
Artymiuk, P. J., and Blake, C. C. F. (1981).J. Mol. Biol. 152, 737–762.
Benton, M. J. (1990).J. Mol. Evol. 30, 409–424.
Bernstein, F. C., Koetzle, T. F., Williams, G. J. B., Meyer, E. F., Brice, M. D., Rodgers, J. R., Kennard, O., Shimanouchi, T., and Tasumi, M. (1977).J. Mol. Biol. 112, 535–542.
Blake, C. C. F., Koenig, D. F., Mair, G. A., North, A. C. T., Phillips, D. C., and Sarma, V. R. (1965).Nature 206, 757–761.
Blake, C. C. F., Mair, G. A., North, A. C. T., Phillips, D. C., and Sarma, V. R. (1967a).Proc. R. Soc. Lond. B 167, 365–377.
Blake, C. C. F., Johnson, L. N., Mair, G. A., North, A. C. T., Phillips, D. C., and Sarma, V. R. (1967b).Proc. R. Soc. Lond. B 167, 378–388.
Brew, K., and Campbell, P. N. (1967).Biochem. J. 102, 258–264.
Brew, K., Vanaman, T. C., and Hill, R. L. (1968).Proc. Natl. Acad. Sci. USA 59, 491–497.
Brew, K., Castellino, F. J., Vanaman, T. C., and Hill, R. L. (1970).J. Biol. Chem. 245, 4570–4582.
Brodbeck, U., and Ebner, K. E. (1966).J. Biol. Chem. 241, 762–764.
Brown, J. R. (1964).Biochem. J. 92, 13P.
Browne, W. J., North, A. C. T., and Phillips, D. C. (1969).J. Mol. Biol. 42, 65–86.
Canfield, R. E. (1963).J. Biol. Chem. 238, 2698–2707.
Canfield, R. E., and Liu, A. K. (1965).J. Biol. Chem. 240, 1997–2002.
Canfield, R. E., Kammerman, S., Sobel, J. H., and Morgan, F. J. (1971).Nature New Biol. 232, 16–17.
Collet, C., Joseph, R., and Nicholas, K. (1990).Reprod. Fertil. Dev. 2, 693–701.
Dautigny, A., Prager, E. M., Pham-Dinh, D., Jollès, J., Pakdel, F., Grindle, B., and Jollès, P. (1991).J. Mol. Evol. 32, 187–198.
Farrell, H. M. Jr., and Thompson, M. P. (1990).Protoplasma 159, 157–167.
Findlay, J. B. C., and Brew, K. (1972).Eur. J. Biochem. 27, 65–86.
Gardiner, B. G. (1982).Zool. J. Linn. Soc. 74, 207–232.
Godovac-Zimmermann, J., Conti, A., and Naptolitano, L. (1988).Biol. Chem. Hoppe-Seyler 369, 1109–1115.
Goodman, M., Miyamoto, M. M., and Czelusniak, J. (1987). InMolecules and Morphology in Evolution: Conflict or Compormise? (Patterson, C., ed.), Cambridge University Press, Cambridge, pp. 141–169.
Grütter, M. G., Weaver, L. H., and Matthews, B. W. (1983).Nature 303, 828–831.
Hall, L., and Campbell, P. N. (1986).Essays Biochem. 22, 1–26.
Hall, L., Craig, R. K., Edbrooke, M. R., and Campbell, P. N. (1982).Nucleic Acids Res. 10, 3503–3515.
Handoll, H. H. G. (1985). D. Phil. Thesis, University of Oxford.
Hermans, J., and McQueen, J. E., Jr. (1974).Acta Crystallogr. A 30, 730–739.
Hiraoka, Y., Segawa, T., Kuwajima, K., Sugai, S., and Murai, N. (1980).Biochem. Biophys. Res. Commun. 95, 1098–1104.
Holpert, M., and Cooper, T. G. (1990).J. Reprod. Fertil. 90, 503–514.
Hopp, T. P., and Woods, K. R. (1979).Biochemistry 18, 5182–5199.
Ibrahimi, I. M., Prager, E. M., White, T. J., and Wilson, A. C. (1979).Biochemistry 18, 2736–2744.
Inaka, K., Kuroki, R., Kikuchi, M., and Matsushima, M. (1991).J. Biol. Chem. 266, 20666–20671.
Irwin, D. M., and Wilson, A. C. (1990).J. Biol. Chem. 265, 4944–4952.
Ito, Y., Yamada, H., Nakamura, M., Yoshikawa, A., Ueda, T., and Imoto, T. (1993).Eur. J. Biochem. 213, 649–658.
Jollès, J., and Jollès, P. (1971).Helv. Chim. Acta 54, 2668–2675.
Jollès, J., Jauregui-Adell, J., Bernier, I., and Jollès, P. (1963).Biochim. Biophys. Acta 76, 668–689.
Jollès, J., van Leemputten, E., Mouton, A., and Jollès, P. (1972).Biochim. Biophys. Acta 257, 497–510.
Jollès, J., Prager, E. M., Alnemri, E. S., Jollès, P., Ibrahimi, I. M., and Wilson, A. C. (1990).J. Mol. Evol. 30, 370–382.
Jones, T. A. (1985). InMethods in Enzymology, Vol. 115 (Wyckoff, H. W., Hirs, C. H. W., and Timasheff, S. N., eds.), Academic Press, Orlando, Florida, pp. 157–171.
Kaminogawa, S., McKenzie, H. A., and Shaw, D. C. (1984).Biochem. Int. 9, 539–546.
Kronman, M. J. (1989).CRC Crit. Rev. Biochem. Mol. Biol. 24, 565–667.
Kuroki, R., Taniyama, Y., Nakamura, H., Kikuchi, M., and Ikehara, M. (1989).Proc. Natl. Acad. Sci. USA 86, 6903–6907.
Linse, S., Brodin, P., Johansson, C., Thulin, E., Grundstrom, T., and Forsen, S. (1988).Nature 335, 651–652.
Marquart, M., Deisenhofer, J., Huber, R., and Palm, W. (1980).J. Mol. Biol. 141, 369–391.
Martin, A. C. R., Cheetham, J. C., and Rees, A. R. (1989).Proc. Natl. Acad. Sci. USA 86, 9268–9272.
McKenna, M. C. (1987). InMolecules and Morphology in Evolution: Conflict or Compromise? (Patterson, C., ed.), Cambridge University Press, Cambridge, pp. 55–93.
McKenzie, H. A., and Shaw, D. C. (1985).Biochem. Int. 10, 23–31.
McKenzie, H. A., and White, F. H., Jr. (1991).Advan. Protein Chem. 41, 173–315.
Moore, A., Hall, L., and Hamilton, D. W. (1990).Biol. Reprod. 43, 497–506.
Musci, G., and Berliner, L. J. (1985).Biochemistry 24, 6945–6948.
Nitta, K., and Sugai, S. (1989).Eur. J. Biochem. 182, 111–118.
Nitta, K., Tsuge, H., Sugai, S. D., and Shimazaki, K. (1987).FEBS Lett. 223, 405–408.
Nitta, K., Tsuge, H., Shimazaki, K., and Sugai, S. D. (1988).Biol. Chem. Hoppe-Seyler 369, 671–675.
Phillips, D. C. (1966).Sci. Am. 215(5), 78–90.
Phillips, D. C., Sternberg, M. J. E., and Sutton, B. J. (1983). InEvolution from Molecules to Man (Bendall, D. S., ed.), Cambridge University Press, Cambridge, pp. 145–173.
Phillips, D. C., Acharya, K. R., Handoll, H. H. G., and Stuart, D. I. (1987).Biochem. Soc. Trans. 15, 737–744.
Prager, E. M., and Wilson, A. C. (1988).J. Mol. Evol. 27, 326–335.
Rao, K. R., and Brew, K. (1989).Biochem. Biophys. Res. Commun. 163, 1390–1396.
Rao, S. T., Hogle, J., and Sundaralingam, M. (1983).Acta Crystallogr. C 39, 237–240.
Rodriguez, R., Mendez-Arias, L., Gonzales de Bruitrago, C., and Gavilanes, J. G. (1985).Biochem. Int. 9, 539–546.
Rodriguez, R., Menedez-Arias, L., Gonzales de Bruitrago, G., and Gavilanes, J. G. (1987).Comp. Biochem. Physiol. 88B, 791–796.
Sali, A., and Blundell, T. L. (1990).J. Mol. Biol. 212, 403–428.
Salton, M. R. J. (1964).The Bacterial Cell Wall, Elsevier, Amsterdam.
Shaw, D. C., Messer, M., Scrivener, A. M., Nicholas, K. R., and Griffiths, M. (1993).Biochim. Biophys. Acta 1161, 177–186.
Shewale, J. G., Sinha, S. K., and Brew, K. (1984).J. Biol. Chem. 259, 4947–4956.
Sibley, C. G., and Ahlquist, J. E. (1990). InThe Phylogeny and Classification of Birds, a Study of Molecular Evolution, Yale University Press, New Haven, Connecticut.
Stuart, D. I., Acharya, K. R., Walker, N. P. C., Smith, S. G., Lewis, M. and Phillips, D. C. (1986).Nature 324, 84–87.
Teahan, C. G., McKenzie, H. A., Shaw, D. C., and Griffiths, M. (1991).Biochem. Int. 24, 85–95.
Thompson, M. P., Groves, M. L., Brower, D. P., Farrell, H. M., Jenness, R., and Kotts, C. E. (1988).Biochem. Biophys. Res. Commun. 157, 944–948.
Thomsen, J., Lund, E. H., Kristiansen, K., Brunfeldt, K., and Malmquist, J. (1972).FEBS Lett. 22, 34–36.
Tsuge, H., Ago, H., Noma, M., Nitta, K., Sugai, S., and Miyano, M. (1992).J. Biochem. 111, 141–143.
Vanaman, T. C., Brew, K., and Hill, R. L. (1970).J. Biol. Chem. 245, 4583–4590.
Vilotte, J.-L., Soulier, S., Mercier, J.-C., Gaye, P., Hue-Delahaie, D., and Furet, J.-P. (1987).Biochimie 69, 609–620.
Warme, P. K., Momany, F. A., Rumball, S. V., Tuttle, R. W., and Scherage, H. A. (1974).Biochemistry 13, 768–782.
White, F. H. Jr., McKenzie, H. A., Shaw, D. C., and Pearce, R. J. (1988).Biochem. Int. 16, 521–528.
Yao, M., Tanaka, I., Hikichi, K., and Nitta, K. (1992).J. Biochem. 111, 1–3.
Zeng, J., Rao, K. R., Brew, K., and Fenna, R. E. (1990).J. Biol. Chem. 265, 14886–14887.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Acharya, K.R., Stuart, D.I., Phillips, D.C. et al. Models of the three-dimensional structures of echidna, horse, and pigeon lysozymes: Calcium-binding lysozymes and their relationship with α-lactalbumins. J Protein Chem 13, 569–584 (1994). https://doi.org/10.1007/BF01901539
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01901539