Abstract
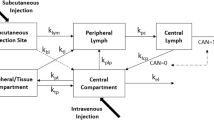
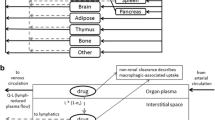
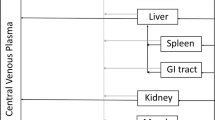
It has been postulated that favouring the absorption of interleukin-2 via lymphatics rather than venous capillaries after subcutaneous adminstration may improve its therapeutic index. We have now evaluated in 12 cancer patients the plasma pharmacokinetic of interleukin-2 either dissolved in water or in 20% albumin solution with an internal cross-over after at least three days. Our data show that when albumin is present, the plasma concentrations of interleukin-2 versus time is increased and swelling at the injection sites is reduced. It remains to be seen whether efficacy improves during a prolonged treatment.
Similar content being viewed by others
Abbreviations
- AUC:
-
Area Under Plasma Curve
- BRM:
-
Biological Response Modifiers
- IFN:
-
Interferon
- IL-2:
-
Interleukin-2
- IV:
-
Intravenous
- SC:
-
Subcutaneous
- MU:
-
Megaunit
References
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, Seipp CA, Simpson CG, White DE. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. New Engl J Med 1987; 316: 889–97.
West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. New Engl J Med 1987; 316: 898–905.
Sarna GP, Figlin RA, Pertcheck M, Altrock B, Kradjian SA. Systemic administration of recombinant methionyl human interleukin-2 (Ala 125) to cancer patients: clinical results. J Biol Resp Modif 1989; 8: 16–24.
Thompson JA, Lee DJ, Cox WW, Lindgren CG, Collins C, Neraas KA, Dennin RA, Fefer A. Recombinant interleukin 2 toxicity, pharmacokinetics, and immunomodulatory effects in a phase I trial. Cancer Res 1987; 47: 4202–7.
Whitehead RP, Ward D, Hemingway L, Hemstreet GP III, Bradley E, Konrad M. Subcutaneous recombinant interleukin-2 in a dose escalating regimen in patients with metastatic renal cell adenocarcinoma. Cancer Res 1990; 50: 6708–15.
Atzpodien J, Körfer A, Franks CR, Poliwoda H, Kirchner H. Home therapy with recombinant interleukin-2 and interferon-α2b in advanced human malignancies. Lancet 1990; 335: 1509–12.
Stein RC, Malkovska V, Morgan S, Galazka A, Aniszewski C, Roy SE, Shearer RJ, Marsden RA, Bevan D, Gordon-Smith EC, Coombes RC. The clinical effects of prolonged treatment of patients with advanced cancer with low-dose subcutaneous interleukin-2. Brit J Cancer 1991; 63: 275–8.
Bocci V. Distribution, catabolism and pharmacokinetics of interferons. In: Finter NB, Oldham RK, eds. Interferon, Vol. 4:in vivo and clinical studies. Elsevier Science Publishers BV, 1985: 47–72.
Bocci V, Pessina GP, Paulesu L, Nicoletti C. The lymphatic route. VI. Distribution of recombinant interferon-α2 in rabbit and pig plasma and lymph. J Biol Resp Modif 1988; 7: 390–400.
Bocci V, Pessina GP, Nicoletti C, Paulesu L. The lymphatic route. VII. Distribution of recombinant human interleukin-2 in rabbit plasma and lymph. J Biol Regul Homeost Agents 1990; 4: 25–9.
Berman M, Weiss MF. SAAM 27. Bethesda, Maryland: National Institute of Health, 1977.
Bocci V. Metabolism of protein anticancer agents. Pharm Ther 1987; 34: 1–49.
Bocci V. Evaluation of routes of administration of interferon in cancer: a review and a proposal. Cancer Drug Del 1984; 1: 337–51.
Oldham RK. Biological response modifiers program. J Biol Resp Modif 1982; 1: 81–100.
Donohue JH, Rosenberg SA. The fate of interleukin-2 afterin vivo adminstration. J Immunol 1983; 130: 2203–8.
Bocci V. Interleukins. Clinical pharmacokinetics and practical implications. Clin Pharmacokinet 1991; 21: 274–84.
Sulis E, Floris C, Massidda C. Intralymphatic infusion of interferon in patients with lymph nodal metastases from melanoma of the lower limbs. Cancer Chemother Pharmacol 1989; 24: 393–4.
Shau H, Isacescu V, Ibayashi Y, Tokuda Y, Golub SH, Fahey JL, Sarna GP. A pilot study of intralymphtic interleukin-2. I. Cytotoxic and surface marker changes of peripheral blood lymphocytes. J Biol Resp Modif 1990; 9: 71–80.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bocci, V., Carraro, F., Zeuli, M. et al. The lymphatic route. VIII. Distribution and plasma clearance of recombinant human interleukin-2 after SC administration with albumin in patients. Biotherapy 6, 73–77 (1993). https://doi.org/10.1007/BF01877388
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01877388