Abstract
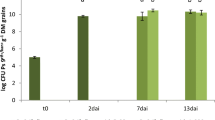
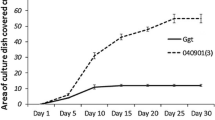
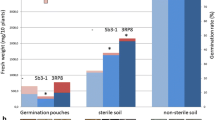
In an earlier study, treatment of radish seed with the bacteriumPseudomonas fluorescens WCS374 suppressed fusarium wilt of radish (Fusarium oxysporum f. sp.raphani) in a commercial greenhouse [Leemanet al., 1991b, 1995a]. In this greenhouse, the areas with fusarium wilt were localized or expanded very slowly, possibly due to disease suppressiveness of the soil. To study this phenomenon, fungi were isolated from radish roots collected from the greenhouse soil. Roots grown from seed treated with WCS374 were more abundantly colonized by fungi than were roots from nonbacterized plants. Among these were several species known for their antagonistic potential. Three of these fungi,Acremonium rutilum, Fusarium oxysporum andVerticillium lecanii, were evaluated further and found to suppress fusarium wilt of radish in a pot bioassay. In an induced resistance bioassay on rockwool,F. oxysporum andV. lecanii suppressed the disease by the apparent induction of systemic disease resistance. In pot bioassays with thePseudomonas spp. strains, the pseudobactin-minus mutant 358PSB− did not suppress fusarium wilt, whereas its wild type strain (WCS358) suppressed disease presumably by siderophore-mediated competition for iron. The wild type strains of WCS374 and WCS417, as well as their pseudobactin-minus mutants 374PSB− and 417PSB− suppressed fusarium wilt. The latter is best explained by the fact that these strains are able to induce systemic resistance in radish, which operates as an additional mode of action. Co-inoculation in pot bioassays, ofA. rutilum, F. oxysporum orV. lecanii with thePseudomonas spp. WCS358, WCS374 or WCS417, or their pseudobactin-minus mutants, significantly suppressed disease (except forA. rutilum/417PSB− and all combinations with 358PSB−), compared with the control treatment, if the microorganisms were applied in inoculum densities which were ineffective in suppressing disease as separate inocula. If one or both of the microorganism(s) of each combination were applied as separate inocula in a density which suppressed disease, no additional suppression of disease was observed by the combination. The advantage of the co-inoculation is that combined populations significantly suppressed disease even when their individual population density was too low to do so. This may provide more consistent biological control. The co-inoculation effect obtained in the pot bioassays suggests that co-operation ofP. fluorescens WCS374 and indigenous antagonists could have been involved in the suppression of fusarium wilt of radish in the commercial greenhouse trials.
Similar content being viewed by others
Abbreviations
- CFU:
-
colony forming units
- KB:
-
King's B
- PGPR:
-
plant growth-promoting rhizobacteria
- CQ:
-
colonization quotient
References
Alabouvette C (1986) Fusarium wilt suppressive soils from the Chateaurenard region: review of a 10 year study. Agronomie 6: 273–284
Baker R (1990) An overview of current and future strategies and models for biological control. In: Hornby D (ed), Biological Control of Soil-Borne Plant Pathogens (pp. 375–389) C.A.B. International, Wallingford
Bakker PAHM, Lamers JG, Bakker AW, Marugg JD, Weisbeek PJ and Schippers B (1986) The role of siderophores in potato tuber yield increase byPseudomonas putida in a short rotation of potato. Neth J Plant Pathol 92: 249–256
Bakker PAHM, Bakker AW, Marugg JD, Weisbeek PJ and Schippers B (1987) Bioassay for studying the role of siderophores in potato growth stimulation byPseudomonas spp. in short potato rotations. Soil Biol Biochem 19: 451–457
Bakker PAHM, Raaijmakers JM and Schippers B (1993) Role of iron in the suppression of bacterial plant pathogens by fluorescent pseudomonads. In: Barton LL and Hemming BC (edS) Iron Chelation in Plants and Soil Microorganisms (pp. 269–278) Academic Press Inc., San Diego
Biles CL and Martyn RD (1989) Local and systemic resistance induced in watermelons by formae speciales ofFusarium oxysporum. Phytopathology 79: 856–860
Couteaudier Y and Alabouvette C (1990) Quantitative comparison ofFusarium oxysporum competitiveness in relation to carbon utilization. FEMS Microbiol Ecol 74: 261–268
Dandurand LM and Knudsen GR (1993) Influence ofPseudomonas fluorescens on hyphal growth and biocontrol activity ofTrichoderma harzianum in the spermosphere and rhizosphere of pea. Phytopathology 83: 265–270
Domsch KH, Gams W and Anderson TH (1980) Compendium of Soil Fungi. Academic Press, London
Duijff BJ, Meijer JW, Bakker PAHM and Schippers B (1993) Siderophore-mediated competition for iron and induced resistance in the suppression of fusarium wilt of carnation by fluorescentPseudomonas spp. Neth J Plant Pathol 99: 277–289
Gams W and Laar W van (1982) The use of solacol (validamycine) as a growth retardant in the isolation of soil fungi. Neth J Plant Pathol 88: 39–45
Gams W, Aa HA van der, Plaats-Niterínk AJ van der, Samson RA and Stalpers JA (1987) CBS Course of Mycology. Centraalbureau voor Schimmelcultures, Baarn, the Netherlands
Geels FP and Schippers B (1983a) Selection of antagonistic fluorescentPseudomonas spp. and their root colonization and persistence following treatment of seed potatoes. J Phytopathol 108:193–206
Geels FP and Schippers B (1983b) Reduction of yield depressions in high frequency potato cropping soil after seed tuber treatments with antagonistic fluorescentPseudomonas spp. J Phytopathol 108:207–214
Geels FP, Schmidt EDL and Schippers B (1985) The use of 8-hydroxyquinoline for the isolation and prequaliflcation of plant growth-stimulating rhizosphere pseudomonads. Biol Fert Soils 1: 167–173
Geels FP, Lamers JG, Hoekstra O and Schippers B (1986) Potato plant response to seed tuber bacterization in the field in various rotations. Neth J Plant Pathol 92: 257–272
Hoagland DR and Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Exp Stn Bull 347: 36–39
Hubbard JP, Harman GE and Hadar Y (1983) Effect of soilbornePseudomonas spp. on the biological control agent,Trichoderma hamatum, on pea seeds. Phytopathology 73: 655–659
King EO, Ward MK and Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307
Kloepper JW and Schroth MN (1978) Plant growth promoting rhizobacteria on radishes. Proceedings of the Fourth International Conference on Plant Pathogenic Bacteria, Angers, France 2: 879–882
Kloepper JW and Schroth MN (1981) Relationship ofin vitro antibiosis of plant growth-promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 71: 1020–1024
Kloepper JW, Leong J, Teintze M and Schroth M (1980)Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4: 317–320
Komada H (1975) Development of a selective medium for quantitative isolation ofFusarium oxysporum from natural soils. Rev Plant Prot Res 8: 114–125
Lamers JG, Schippers B and Geels FP (1988) Soil-borne diseases of wheat in the Netherlands and results of seed bacterization with pseudomonads againstGaeumannomycesgraminis var.tritici. In: Jorna ML and Slootmaker LAJ (eds) Cereal Breeding Related to Integrated Cereal Production (pp. 134–139) Pudoc, Wageningen
Leeman M (1994) Suppression of fusarium wilt of radish by fluorescentPseudomonas spp. — Induction of disease resistance, co-inoculation with fungi and commercial application. Ph.D. thesis, Utrecht University, Utrecht
Leeman M, Raaijmakers JM, Bakker PAHM and Schippers B (1991a) Immunofluorescence colony staining for monitoring pseudomonads introduced into soil. In: Beemster ABR, Bollen GJ, Gerlagh M, Ruissen MT, Schippers B and Tempel A (eds) Biotic Interactions and Soil-Borne Diseases (pp. 374–380) Elsevier, Amsterdam
Leeman M, Scheffer RJ, Pelt JA van, Bakker PAHM and Schippers B (1991b) Control of fusarium wilt of radish byPseudomonas fluorescens WCS374, in greenhouse trials. In: Keel C, Koller B and Défago G (eds) Plant Growth-Promoting Rhizobacteria: Progress and Prospects, West Palaearctic Reg Sec Bull Vol 14/8 (pp. 34–38) Int Org Biol Integrated Control Noxious Anim Plants
Leeman M, Pelt JA van, Hendrickx MJ, Scheffer RJ, Bakker PAHM and Schippers B (1995a) Biocontrol of fusarium wilt of radish in commercial greenhouse trials by seed treatment withPseudomonas fluorescens WCS374. Phytopathology: in press
Leeman M, Pelt JA van, Ouden FM den, Heinsbroek M, Bakker PAHM and Schippers B (1995b) Induction of systemic resistance byPseudomonas fluorescens in radish cultivars differing in susceptibility to fusarium wilt, using a novel bioassay. Europ J Plant Pathol: in press
Leeman M, Pelt JA van, Ouden FM den, Heinsbroek M, Bakker PAHM and Schippers B (1995c) Induction of systemic resistance against fusarium wilt of radish by lipopolysaccharides ofPseudomonas fluorescens. Phytopathology: in press
Lemanceau P and Alabouvette C (1991) Biological control of fusarium diseases by fluorescentPseudomonas and non-pathogenicFusarium Crop Prot. 10: 279–286
Lemanceau P and Alabouvette C (1993) Suppression of fusarium wilts by fluorescent pseudomonads: mechanisms and applications. Biocontrol Sc Techn 3: 219–234
Lemanceau P, Bakker PAHM, Kogel WJ de, Alabouvette C and Schippers B (1992) Effect of pseudobactin 358 production byPseudomonas putida WCS358 on suppression of fusarium wilt of carnations by nonpathogenicFusarium oxysporum Fo47. Appl Environ Microbiol 58: 2978–2982
Lemanceau P, Bakker PAHM, Kogel WJ de, Alabouvette C and Schippers B (1993) Antagonistic effect of nonpathogenicFusarium oxysporum Fo47 and pseudobactin 358 upon pathogenicFusarium oxysporum f. sp.dianthi. Appl Environ Microbiol 59: 74–82
Louvet J, Alabouvette C and Rouxel F (1981) Microbiological suppressiveness of some soils to fusarium wilts. In: Nelson PE, Toussoun TA and Cook RJ (eds) Fusarium: Diseases, Biology and Taxonomy (pp. 261–275) Pennsylvania State University Press
Mandeel Q and Baker R (1991) Mechanisms involved in biological control of fusarium wilt of cucumber with strains of nonpathogenicFusarium oxysporum. Phytopathology 81: 462–469
Marugg JD, Spanje M van, Hoekstra WPM, Schippers B and Weisbeek PJ (1985) Isolation and analysis of genes involved in siderophore biosynthesis in plant growth-stimulatingPseudomonas putida WCS358. J Bacteriol 164: 563–570
Miller RH and May S (1991) Legume inoculation: successes and failures. In: Keister DL and Cregan PB (eds) The Rhizosphere and Plant Growth (pp. 123–135) Kluwer Academic Publishers, Dordrecht
Park CS, Paulitz TC and Baker R (1988) Biocontrol of fusarium wilt of cucumber resulting from interactions betweenPseudomonas putida and non-pathogenic isolates ofFusarium oxysporum. Phytopathology 78: 190–194
Paulitz TC, Park CS and Baker R (1987) Biological control of fusarium wilt of cucumber with non pathogenic isolates ofFusarium oxysporum. Can J Microbiol 33: 349–353
Postma J and Rattink H (1992) Biological control of fusarium wilt of carnation with a nonpathogenic isolate ofFusarium oxysporum. Can J Bot 70: 1199–1205
Raaijmakers JM (1994) Microbial interactions in the rhizosphere — Root colonization byPseudomonas spp. and suppression of fusarium wilt. Ph.D. thesis, Utrecht University, Utrecht
Raaijmakers JM, Sluis I van der, Koster M, Bakker PAHM, Weisbeek PJ and Schippers B (1995) Utilization of heterologous siderophores and rhizosphere competence of fluorescentPseudomonas spp. Can J Microbiol: 41: 126–135
Scher FM and Baker R (1980) Mechanism of biological control in a fusarium-suppressive soil. Phytopathology 70: 412–417
Scher FM and Baker R (1982) Effect ofPseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to fusarium wilt pathogens. Phytopathology 72: 1567–1573
Schippers B (1992) Prospects for management of natural suppressiveness to control soilborne pathogens. In: Tjamos EC, Papavizas GC and Cook RJ (eds) Biological Control of Plant Diseases, Progress and Challenges for the Future, NATO ASI Series, Series A: Life Sciences. Vol. 230 (pp. 21–34) Plenum Press, New York and London
Sneh B, Dupler M, Elad Y and Baker R (1984) Chlamydospore germinationof Fusarium oxysporum f.sp.cucumerinum as affected by fluorescent and lytic bacteria from Fusarium-suppressive soil. Phytopathology 74: 1115–1124
Van Peer R and Schippers B (1989) Plant growth responses to bacterization and rhizosphere microbial development in hydroponic cultures. Can J Microbiol 35: 456–463
Van Peer R and Schippers B (1992) Lipopolysaccharides of plant growth-promotingPseudomonas sp. strain WCS417r induce resistance in carnation to fusarium wilt. Neth J Plant Pathol 98: 129–139
Van Peer R, Kuik AJ van, Rattink H and Schippers B (1990) Control of fusarium wilt of carnation grown on rockwool byPseudomonas sp. strain WCS417r and by FeEDDHA. Neth J Plant Pathol 96: 119–132
Van Peer R, Niemann GJ and Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation byPseudomonas fluorescens strain WCS417r. Phytopathology 81: 728–734
Van Vuurde JWL and Roozen NJM (1990) Comparison of immunofluorescence colony-staining in media, selective isolation on pectate medium, ELISA and immunofluorescence cell staining for detection ofErwinia carotovora subsp.atroseptica andE. chrysanthemi in cattle manure slurry. Neth J Plant Pathol 96: 75–89
Weisbeek PJ, Hofstad GAJM van der, Schippers B and Marugg JD (1986) Genetic analysis of the iron-uptake system of two plant growth-promotingPseudomonas strains. In: Swinburne TR (ed) Iron, Siderophores and Plant Diseases (pp. 299–313) Plenum Press, New York
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26: 379–407
Weller DM and Cook RJ (1983) Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463–469
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leeman, M., Den Ouden, F.M., Van Pelt, J.A. et al. Suppression of fusarium wilt of radish by co-inoculation of fluorescentPseudomonas spp. and root-colonizing fungi. Eur J Plant Pathol 102, 21–31 (1996). https://doi.org/10.1007/BF01877112
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01877112