Abstract
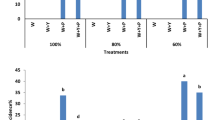
Cell suspension cultures were set up from two tomato cultivars, one resistant, (‘Rio grande’) and one susceptible (‘63.5’) toFusarium oxysporum f. sp.lycopersici. Growth rates of the two cell cultures were comparable. Toxicity of fusaric acid, expressed as the fresh weight loss, was analyzed: It was significant in both cases after 10 h, but toxicity was twice as high for ‘63.5’ suspension cells. In the same way, electrolyte leakage caused by fusaric acid was three times more important for ‘63.5’ suspension cells. Moreover, fusaric acid treatment resulted in an acidification of the extracellular medium for ‘63.5’ suspension cells (0.4 pH unit), whereas an alkalization was observed for ‘Rio grande’ suspension cells (0.2 pH unit). Preliminary experiments suggest that fusaric acid was partially metabolized by ‘Rio grande’ suspension cells, however, no detoxified forms of fusaric acid were detected either in cells or in culture filtrates. For these two tomato cultivars, the differences in sensitivity to fusaric acid of cultivated cells correspond to the differences in plant susceptibility toFusarium oxysporum f. sp.lycopersici.
Similar content being viewed by others
Abbreviations
- BAP:
-
6-benzylaminopurine
- γ :
-
conductivity
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- EtOAc:
-
ethyl acetate
- FA:
-
fusaric acid
- ρ :
-
resistivity
References
Barna B, Sarhan ART and Kiràly Z (1983) The influence of nitrogen nutrition on the sensitivity of tomato plants to culture filtrates ofFusarium and to fusaric acid. Physiol Plant Pathol 23: 257–263
Chandler MT, Tandeau de Marsac N and de Kouchovsky Y (1972) Photosynthetic growth of tobacco cells in liquid suspension. Can J Bot 50: 2265–2270
Chakrabarti DK and Basu Chaudhary KC (1980) Correlation between virulence and fusaric acid production inFusarium oxysporum f. sp.carthami. Phytopath Z 99: 43–46
D'Alton A and Etherton B (1984) Effects of fusaric acid on tomato root hair membrane potentials and ATP levels. Plant Physiol 74: 39–42
Davis D (1969) Fusaric acid in selective pathogenicity ofFusarium oxysporum. Phytopathology 59: 1391–1395
Dunkle LD and Wolpert TJ (1981) Independence of milo disease symptoms and electrolyte leakage induced by the host-specific toxin fromPericonia circinata. Physiol Plant Pathol 18: 315–323
GÄumann E (1958) The mechanisms of fusaric acid injury. Phytopathology 48: 670–686
Jullien M (1988) Effects of theFusarium sp. toxins and selection of crude toxin resistant strains in mesophyll cell cultures ofAsparagus officinalis. Plant Physiol Biochem 26: 713–721
Kluepfel D (1957) über die Biosynthese und die Umwandlungen der FusarinsÄure in Tomatenpflanzen. Phytopathol Z 29: 349–379
Köhler K and Bentrup F-W (1983) The effect of fusaric acid upon electrical membrane properties and ATP level in photoautotrophic cell suspension cultures ofChenopodium rubrum. L Z Pflanzenphysiol 109: 355–361
Kuo MS and Scheffer RP (1964) Evaluation of fusaric acid as a factor in development of Fusarium wilt. Phytopathology 54: 1041–1044
Linskens HF (1955) Der Einflu\ der toxigenen Welke auf die Blat-tausscheidungen der Tomatenpflanze. Phytopathol Z 23: 89–106
Marrè MT, Vergani P and Albergoni FG (1993) Relationship between fusaric acid uptake and its binding to cell structures by leaves ofEgeria densa and its toxic effects on membrane permeability and respiration. Physiol Mol Plant Pathol 42: 141–157
Mégnégneau B and Branchard M (1988) Toxicity of fusaric acid observed on callus cultures of variousCucumis melo genotypes. Plant Physiol Biochem 26: 585–588
Shahin EA and Spivey R (1986) A single dominant gene forFusarium wilt resistance in protoplast-derived tomato plants. Theor Appl Genet 73: 164–169
Storti E, Latil C, Salti S, Bettini P, Bogani P, Pellegrini M G, Simeti C, Molnar A and Buatti M (1992) Thein vitro physiological phenotype of tomato resistance toFusarium oxysporum f. sp.lycopersici. Theor Appl Genet 84: 123–128
Tisserat B (1985) Embryogenesis, organogenesis and plant regeneration. In: Dixon RA (ed) Plant cell culture: a practical approach. Vol. 1 (pp. 79–105) IRL Press, Oxford
Toyoda H, Hashimoto H, Utsumi R, Kobayashi H and Ouchi S (1988) Detoxification of fusaric acid by a fusaric acid-resistant mutant ofPseudomonas solanacearum and its application to biological control of Fusarium wilt of tomato. Phytopathology 78: 1307–1311
Zaichenko AM and Bogomolova LA (1984) Effect of certain mycotoxins on enzymic activity of carbohydrate metabolism inDendrodochium Toxicum. Mikrobiol Z 46: 38–42
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gapillout, I., Milat, M.L. & Blein, J.P. Effects of fusaric acid on cells from tomato cultivars resistant or susceptible toFusarium oxysporum f. sp.Lycopersici . Eur J Plant Pathol 102, 127–132 (1996). https://doi.org/10.1007/BF01877099
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01877099