Summary
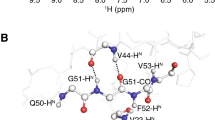
Non-glycine residues with positive φ-angles have been identified in four proteins, barley serine proteinase inhibitor CI-2, bacterial ribonuclease (barnase) ofBacillus amyloliquefaciens, hen egg white lysozyme and a basic protein from barley seed (barwin) by use of nuclear magnetic resonance spectroscopy. By accurate measurements of the coupling constant\({}^3J_{N^N H^2 } \) and integration of the nuclear Overhauser HN-Hα cross peak, positive φ-angles could be determined reliably to 60°±30°, in full agreement with the crystal structures for lysozyme, barnase and serine proteinase inhibitor CI-2. The work emphasizes that positive φ-angles can also occur in non-glycine residues and in the four proteins, positive φ-angles have been observed for the residue types aspartic acid, asparagine, arginine, serine, glutamine, histidine, tyrosine, tryptophan and phenylalanine. The measured\({}^3J_{N^N H^2 } \) coupling constants and the intensity of the intraresidue HN-Hα NOEs agree well with the solution structures of three of the proteins, using the existing parametrization of the Karplus curve (Pardi, A., Billeter, M. and Wüthrich, K. (1984)J. Mol. Biol.,180, 741–751; Ludvigsen, S., Andersen, K.V. and Poulsen, F.M. (1991)J. Mol. Biol.,217, 731–736).
Similar content being viewed by others
References
Anil-Kumar, Ernst, R.R. and Wüthrich, K. (1980)Biochem. Biophys. Res. Commun.,95, 1–6.
Anil-Kumar, Wagner, G., Ernst, R.R. and Wüthrich, K. (1981)J. Am. Chem. Soc. 103, 3654–3658.
Bycroft, M., Sheppard, R.N., Lau, S.T.K. and Fersht, A.R. (1990)Biochemistry,29, 7425–7432.
Bycroft, M., Ludvigsen, S., Fersht, A.R. and Poulsen, F.M. (1991)Biochemistry,30, 8697–8701.
Clore, G.M., Gronenborn, A.M., Kjær, M. and Poulsen, F.M. (1987)Prot. Eng.,1, 305–311.
Clore, G.M., Wingfield, P.T. and Gronenborn, A.M. (1991)Biochemistry.30, 2315–2323.
Crawford, J.L., Lipscomb, W.N. and Schellman, C.G. (1973)Proc. Natl. Acad. Sci. USA.70, 538–542.
Handoll (1985) Ph. D. Thesis, Oxford University.
Jeener, J., Meier, B.H., Bachmann, P. and Ernst, R.R. (1979)J. Chem. Phys.,71, 4546–4553.
Karplus, M. (1959)J. Phys. Chem.,30, 11–15.
Kjær M., Ludvigsen, S., Sørensen, O.W., Denys, L.A., Kindtler, J. and Poulsen, F.M. (1987)Carlsberg Res. Commun. 52, 327–354.
Kjær M., Andersen, K.V., Ludvigsen, S., Shen, H., Windekilde, D., Sørensen, B. and Poulsen, F.M. (1991) InComputational Aspects of the Study of Biological Macromolecules (Eds, Hoch, J.C., Poulsen, F.M. and Redfield, C.) NATO ASI Series,Ser. A,225, 291–302.
Kline, A.D., Braun, W. and Wüthrich, K. (1988)J. Mol. Biol.,204, 675–724.
Lewis, P.N., Momany, F.A., and Scheraga, H.A. (1973)Biochim. Biophys. Acta,303, 211–229.
Ludvigsen, S., Andersen, K.V. and Poulsen, F.M. (1991a)J. Mol. Biol.,217, 731–736.
Ludvigsen, S., Shen, H., Kjær, M., Madsen, J.C. and Poulsen, F.M. (1991b)J. Mol. Biol.,222, 621–635.
Ludvigsen, S. and Poulsen, F.M. (1992a), submitted for publication.
Ludvigsen, S. and Poulsen, F.M. (1992b), submitted for publication.
Mauguen, Y., Hartley, R.W., Dodson, E.J., Dodson, G.G., Bricogne, G., Chothia, C. and Jack, A. (1982)Nature,297, 162–164.
McPhalen, C.A. and James, M.N.G. (1987)Biochemistry,26, 261–269.
Nicholson, H., Söderlind, E., Tronrud, D.E. and Matthews, B.W. (1989)J. Mol. Biol.,210, 181–193.
Overington, J., Johnson, M.S., Sali, A. and Blundell, T.L. (1990)Proc. Roy. Soc. Lond. B.,241, 132–145.
Pardi, A., Billeter, M. and Wüthrich, K. (1984)J. Mol. Biol.,180, 741–751.
Piantini, U., Sørensen, O.W. and Ernst, R.R. (1982)J. Am. Chem. Soc.,104, 6800–6801.
Rance, M., Sørensen, O.W., Bodenhausen, G., Wagner, G., Ernst, R.R. and Wüthrich, K. (1983)Biochem. Biophys. Res. Commun.,117, 479–485.
Redfield, C. and Dobson, C.M. (1988)Biochemistry,27, 122–136.
Shen, H. and Poulsen, F.M. (1990)J. Magn. Reson.,89, 585–594.
Smith, L.J., Sutcliffe, M.J., Redfield, C. and Dobson, C.M. (1991)Biochemistry,30, 986–996.
Venkatachalam, C.M. (1968)Biopolymers,6, 1425–1436.
Wilmot, C.M. and Thornton, J.M. (1990)Prot. Eng.,3, 479–493.
Wüthrich, K. (1986)NMR of Proteins and Nucleic Acids, Wiley-Interscience, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ludvigsen, S., Poulsen, F.M. Positive φ-angles in proteins by nuclear magnetic resonance spectroscopy. J Biomol NMR 2, 227–233 (1992). https://doi.org/10.1007/BF01875318
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01875318