Summary
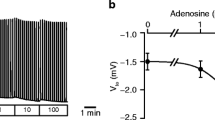
We have examined the effects of various inositol polyphosphates, alone and in combination, on the Ca2+-activated K+ current in internally perfused, single mouse lacrimal acinar cells. We used the patch-clamp technique for whole-cell current recording with a set-up allowing exchange of the pipette solution during individual experiments so that control and test periods could be directly compared in individual cells. Inositol 1,4,5-trisphosphate (Ins 1,4,5 P3) (10–100 μm) evoked a transient increase in the Ca2+-sensitive K+ current that was independent of the presence of Ca2+ in the external solution. The transient nature of the Ins 1,4,5 P3 effect was not due to rapid metabolic breakdown, as similar responses were obtained in the presence of 5mm 2,3-diphosphoglyceric acid, that blocks the hydrolysis of Ins 1,4,5 P3, as well as with the stable analoguedl-inositol 1,4,5-trisphosphorothioate (Ins 1,4,5 P(S)3) (100 μm). Ins 1,3,4 P3 (50 μm) had no effect, whereas 50 μm Ins 2,4,5 P3 evoked responses similar to those obtained by 10 μm Ins 1,4,5 P3. A sustained increase in Ca2+-dependent K+ current was only observed when inositol 1,3,4,5-tetrakisphosphate (Ins 1,3,4,5 P4) (10 μm) was added to the Ins 1,4,5 P3 (10 μm)-containing solution and this effect could be terminated by removal of external Ca2+. The effect of Ins 1,3,4,5 P4 was specifically dependent on the presence of Ins 1,4,5 P3 as it was not found when 10 μm concentrations of Ins 1,3,4 P3 or Ins 2,4,5 P3 were used. Ins 2,4,5 P3 (but not Ins 1,3,4 P3) at the higher concentration of 50 μm did, however, support the Ins 1,3,4,5 P4-evoked sustained current activation. Ins 1,3,4 P3 could not evoke sustained responses in combination with Ins 1,4,5 P3 excluding the possibility that the action of Ins 1,3,4,5 P4 could be mediated by its breakdown product Ins 1,3,4 P3. Ins 1,3,4,5 P4 also evoked a sustained response when added to an Ins 1,4,5 P(S)3-containing solution. Ins 1,3,4,5,6 P5 (50 μm) did not evoke any effect when administered on top of Ins 1,4,5 P3. In the absence of external Ca2+, addition of Ins 1,3,4,5 P4 to an Ins 1,4,5 P3-containing internal solution evoked a second transient K+ current activation. Readmitting external Ca2+ in the continued presence internally of Ins 1,4,5 P3 and Ins 1,3,4,5 P4 made the response reappear. We conclude that both Ins 1,4,5 P3 and Ins 1,3,4,5 P4 play crucial and specific roles in controlling intracellular Ca2+ homeostasis.
Similar content being viewed by others
References
Batty, I.R., Nahorski, S.R., Irvine, R.F. 1985. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices.Biochem. J. 232:211–215
Berridge, M.J. 1988. Inositol lipids and calcium signalling.Proc. R. Soc. London B. 234:359–378
Berridge, M.J., Irvine, R.F. 1984. Inositol trisphosphate, a novel second messenger in cellular signal transduction.Nature (London) 312:315–321
Biden, T.J., Wollheim, C.B. 1986. Ca2+ regulates the tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells.J. Biol. Chem. 261:11931–11934
Bradford, P.G., Irvine, R.F. 1987. Specific binding sites for [3H]-inositol (1,3,4,5) tetrakisphosphate on membranes of HL-60 cells.Biochem. Biophys. Res. Commun. 149:680–685
Burgen, A.S.V. 1956. The secretion of potassium in saliva.J. Physiol. (London) 132:20–39
Connolly, T.M., Bansal, V.S., Bross, T.E., Irvine, R.F., Majerus, P.W. 1987. The metabolism of tris and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes.J. Biol. Chem. 262:2146–2149
Cooke, A.M., Gigg, R., Potter, B.V.L. 1987. Myo-inositol 1,4,5-trisphosphorothioate: A novel analogue of a biological second messenger.J. Chem. Soc. Commun. 262:1525–1526
Cooke, A.M., Nahorski, S.R., Potter, B.V.L. 1989. Myo-inositol 1,4,5-trisphosphorothioate is a potent competitive inhibitor of human erythrocyte 5-phosphatase.FEBS Lett. 242:373–377
Crossley, I., Swann, K., Chambers, E., Whitaker, M. 1988. Activation of sea urchin eggs is independent of external calcium ions.Biochem. J. 252:257–262
Downes, C.P., Mussat, M.C., Michell, R.H. 1982. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane.Biochem. J. 203:169–177
Enyedi, P., Williams, G.H. 1988. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex.J. Biol. Chem. 263:7940–7942
Findlay, I. 1984. A patch-clamp study of potassium channels and whole-cell currents in acinar cells of the mouse lacrimal gland.J. Physiol. (London) 350:179–195
Gallacher, D.V. 1988. Control of calcium influx in cells without action potentials.News Physiol. Sci. 3:244–249
Gallacher, D.V., Morris, A.P. 1986. A patch-clamp study of potassium currents in resting and acetylcholine-stimulated mouse submandibular acinar cells.J. Physiol. (London) 373:379–395
Gallacher, D.V., Morris, A.P. 1987. The receptor-regulated calcium influx in mouse submandibular acinar cells is sodium dependent: A patch-clamp study.J. Physiol. (London) 334:119–130
Hamblin, M.R., Flora, J.S., Potter, B.V.L. 1987. Phosphorothioates, phosphatase-resistant analogues of myo-inositol phosphates. Synthesis ofdl-myoinositol 1,4 bisphosphate anddl-myo-inositol 1,4 bisphosphorothioate.Biochem. J. 246:771–774
Hamill, O.P., Marty, A., Neher, E., Sakmann, B., Sigworth, F.J. 1981. Improved patch-clamp techniques for high resolution current recordings from cells and cell-free membrane patches.Pfluegers Arch. 391:85–100
Hawkins, P.T., Stephens, L., Downes, C.P. 1986. Rapid formation of inositol (1,3,4,5) tetrakisphosphate and inositol (1,3,4) trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol (1,4,5) trisphosphate from phosphatidylinositol 4,5-bisphosphate.Biochem. J. 238:507–516
Hill, T.D., Dean, N.M., Boynton, A.L. 1988. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells.Science 242:1176–1178
Irvine, R.F., Letcher, A.J., Heslop, J.P., Berridge, M.J. 1986a. The inositol tris/tetrakisphosphate pathway-demonstration of Ins (1,4,5) P3-3-kinase activity in animal tissues.Nature (London) 320:631–634
Irvine, R.F., Letcher, A.J., Lander, D.J., Berridge, M.J. 1986b. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells.Biochem. J. 240:301–304
Irvine, R.F., Moor, R.M. 1986. Microinjection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+.Biochem. J. 240:917–920
Irvine, R.F., Moor, R.M. 1987. Inositol (1,3,4,5) tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate.Biochem. Biophys. Res. Commun. 146:284–290
Irvine, R.F., Moor, R.M., Pollock, W.K., Smith, P.M., Wreggett, K.A. 1988. Inositol phosphates: Proliferation, metabolism and function.Phil. Trans. R. Soc. London B 320:281–298
Jauch, P., Petersen, O.H., Läuger, P. 1986. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings.J. Membrane Biol. 94:99–115
Joseph, S.K., Hansen, C.A., Williamson, J.R. 1987. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient.FEBS Lett. 214:125–129
Llano, I., Marty, A., Tanguy, J. 1987. Dependence of intracellular effects of GTP γ S and inositoltrisphosphate on cell membrane potential and on external Ca ions.Pfluegers Arch. 409:499–506
Maruyama, Y., Petersen, O.H. 1984. Control of K+ conductance by cholecystokinin and Ca2+ in single pancreatic acinar cells studied by the patch-clamp technique.J. Membrane Biol. 79:293–300
Maruyama, Y., Petersen, O.H., Flanagan, P., Pearson, G.T. 1983. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells.Nature (London) 305:228–232
Morris, A.P., Fuller, C.M., Gallacher, D.V. 1987a. Cholinergic receptors regulate a voltage-insensitive but Na+-dependent calcium influx pathway in salivary acinar cells.FEBS Lett. 211:195–199
Morris, A.P., Gallacher, D.V., Fuller, C.M., Scott, J. 1987b. Cholinergic receptor-regulation of potassium channels and potassium transport in human submandibular acinar cells.J. Dent. Res. 66:541–546
Morris, A.P., Gallacher, D.V., Irvine, R.F., Petersen, O.H. 1987c. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K− channels.Nature (London) 330:653–655
Mullaney, J.M., Chueh, S.-H., Ghosh, T.K., Gill, D.L. 1987. Intracellular calcium uptake activated by GTP: Evidence for a possible guanine nucleotide-induced transmembrane conveyance of intracellular calcium.J. Biol. Chem. 262:13865–13872
Nauntofte, B., Dissing, S. 1987. Stimulation-induced changes in cytosolic calcium in rat parotid acini.Am. J. Physiol. 253:G290-G297
Nishizuka, Y. 1986. Studies and perspectives of protein kinase C.Science 233:305–312
Ohya, Y., Terada, K., Yamaguchi, K., Inoue, R., Okabe, K., Kitamura, K., Hirata, M., Kuriyama, H. 1988. Effects of inositol phosphates on the membrane activity of smooth muscle cells of the rabbit portal vein.Pfluegers Arch. 412:382–389
Parker, I., Miledi, R. 1987. Injection of inositol 1,3,4,5-tetrakisphosphate intoXenopus oocytes generates a chloride current dependent upon intracellular calcium.Proc. R. Soc. London B. 232:59–70
Penner, R., Matthews, G., Neher, E. 1988. Regulation of calcium influx by second messengers in rat mast cells.Nature (London) 334:449–504
Petersen, O.H. 1986. Calcium-activated potassium channels and fluid secretion by exocrine glands.Am. J. Physiol. 251:G1-G13
Petersen, O.H., Findlay, I., Iwatsuki, N., Singh, J., Gallacher, D.V., Fuller, C.M., Pearson, G.T., Dunne, M.J., Morris, A.P. 1985. Human pancreatic acinar cells: Studies of stimulus-secretion coupling.Gastroenterology 89:109–117
Petersen, O.H., Gallacher, D.V. 1988. Electrophysiology of pancreatic and salivary acinar cells.Annu. Rev. Physiol. 50:65–80
Petersen, O.H., Maruyama, Y. 1984. Calcium-activated potassium channels and their role in secretion.Nature (London) 307:693–696
Snyder, P.M., Krause, K.-H., Welsh, M.J. 1988. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate induce calcium influx inXenopus laevis oocytes.J. Biol. Chem. 263:11048–11051
Streb, H., Bayerdörffer, E., Haase, W., Irvine, R.F., Schulz, I. 1984. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas.J. Membrane Biol. 81:241–253
Streb, H., Irvine, R.F., Berridge, M.J., Schulz, I. 1983. Refease of Ca2+ from a non-mitochondrial intracellular store of pancreatic acinar cells by inositol 1,4,5 trisphosphate.Nature (London) 306:67–69
Suzuki, K., Petersen, C.C.H., Petersen, O.H. 1985. Hormonal activation of single K+ channels via internal messenger in isolated pancreatic acinar cells.FEBS Lett. 192:307–312
Suzuki, K., Petersen, O.H. 1988. Patch-clamp study of single-channel and whole-cell K+ currents in guinea pig pancreatic acinar cells.Am. J. Physiol. 255:G275-G285
Taylor, C.W., Berridge, M.J., Brown, K.D., Cooke, A.M., Potter, B.V.L. 1988.dl-myo-inositol 1,4,5-trisphosphorothioate mobilizes intracellular calcium in Swiss 3T3 cells andXenopus oocytes.Biochem. Biophys. Res. Commun. 150:626–632
Thévenod, F., Dehlinger-Kremer, M., Kemmer, T.P., Christian, A.-L., Potter, B.V.L., Schulz, I. 1989. Characterization of inositol 1,4,5-trisphosphate-sensitive (IsCaP) and-insensitive (IisCaP) nonmitochondrial Ca2+ pools in rat pancreatic acinar cells.J. Membrane Biol. (in press)
Trautmann, A., Marty, A. 1984. Activation of Ca-dependent K channels by carbamylcholine in rat lacrimal glands.Proc. Natl. Acad. Sci. USA 81:611–615
Trimble, E.R., Bruzzone, R., Meehan, C.J., Biden, T.J. 1987. Rapid increases in inositol 1,4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate and cytosolic free Ca2+ in agonist-stimulated pancreatic acini of the rat.Biochem. J. 242:289–292
Volpe, P., Krause, K.-H., Hashimoto, S., Zorgato, F., Pozzan, T., Meldolesi, J., Lew, D.P. 1988. ‘Calciosome’, a cytoplasmic organelle: The inositol 1,4,5-trisphosphate-sensitive Ca2+ store of non-muscle cells.Proc. Natl. Acad. Sci. USA 85:1091–1095
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Changya, L., Gallacher, D.V., Irvine, R.F. et al. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J. Membrain Biol. 109, 85–93 (1989). https://doi.org/10.1007/BF01870793
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870793