Summary
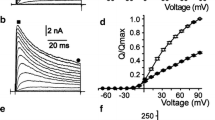
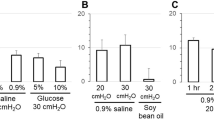
Several new amiloride analogues and two reported photoaffinity analogues were tested for irreversible inhibition of short-circuit current,I sc, in toad bladder. Bromoamiloride, a photoaffinity analogue, induced 40% irreversible inhibition at 500 μm after irradiation with ultraviolet light ≥320 nm. Iodoamiloride caused no irreversible inhibition. Of the new analogues tested, only 3,5-diamino-6-chloro-N-[(phenylamino) aminomethylene] pyrazinecarboxamide,phenamil, irreversibly inhibitedI sc at concentrations of 0.05 to 5 μm when added to the mucosal solution. Irreversible inhibition ofI sc by phenamil may be attributed to specific blockage of the mucosal sodium channels, which depended on: 1) time of exposure; 2) mucosal pH: 3) mucosal sodium concentration. For example, 5 μm phenamil irreversibly inhibitedI sc by 38% in 103mm Na at pH 8.6 and nearly 75% in 30mm Na at pH 6.4 after a 40-min exposure. Irreversible inhibition occurred in two phases with time constants of ≤10 min and approximately 140 min. Due to its irreversible nature, phenamil may be used to measure channel density.
Similar content being viewed by others
References
Benos, D.J. 1982. Amiloride: A molecular probe of sodium transport in tissues and cells.Am. J. Physiol. 242:C131-C145
Benos, D.J., Hyde, B.A., Latorre, R. 1983. Sodium flux ratio through the amiloride-sensitive entry pathway in frog skin.J. Gen. Physiol. 81:667–685
Benos, D.J., Mandel, L.J. 1978. Irreversible inhibition of sodium entry sites in frog skin by a photosensitive amiloride analog.Science 199:1205–1206
Benos, D.J., Mandel, L.J., Balaban, R.S. 1979. On the mechnism of the amiloride-sodium entry site interaction in anuran skin epithelia.J. Gen. Physiol. 73:307–326
Benos, D.J., Mandel, L.J., Simon, S.A. 1980. Effects of chemical group specific reagents on sodium entry and the amiloride binding site in frog skin: Evidence for separate sites.J. Membrane Biol. 56:149–158
Benos, D.J., Simon, S.A., Mandel, L.J., Cala, P.M. 1976. Effect of amiloride and some of its analogues on cation transport in isolated frog skin and thin lipid membranes.J. Gen. Physiol. 68:43–63
Bentley, P.J. 1968. Amiloride: A potent inhibitor of sodium transport across the toad bladder.J. Physiol. (London) 195:317–330
Cobb, M.H., Scott, W.N. 1981. Irreversible inhibition of sodium transport by the toad urinary bladder following photolysis of amiloride analogs.Experientia 37:68–69
Cragoe, E.J., Jr. 1979. Structure-activity relationships in the amiloride series.In: Amiloride and Epithelial Sodium Transport. A.W. Cuthbert, G.M. Fanelli and A. Scriabine, editors. pp. 1–20. Urban and Schwarzenberg, Baltimore, Md.
Cragoe, E.J., Jr., Woltersdorf, O.W., Jr., Bicking, J.B., Kwong, S.F., Jones, J.W. 1967. Pyrazine diuretics. II. N-Amidino-3-amino-5-substituted-6-halopyrazinecarboxamides.J. Med. Chem. 10:66
Cuthbert, A.W. 1976. Importance of guanididium groups for blocking sodium channels in epithelia.Mol. Pharmacol. 12:945–957
Cuthbert, A.W., Shum, W.K. 1974. Binding of amiloride to sodium channels in frog skin.Mol. Pharmacol. 10:880–891
Garty, H., Edelman, I.S. 1983. Amiloride-sensitive trypsinization of apical sodium channels.J. Gen. Physiol. 81:785–803
MacKnight, A.D.C., DiBona, D.R., Leaf, A. 1980. Sodium transport across toad urinary bladder: A model “tight” epithelium.Physiol. Rev. 60:615–715
Mandel, L.J., Curran, P.F. 1973. Response of the frog skin to steady-state voltage clamping. II. The active pathway.J. Gen. Physiol. 62:1–24
Morrison, R.T., Boyd, R.N. 1973. Organic Chemistry. 3rd ed. pp. 452–491, Allyn and Bacon, Boston, Mass.
Park, C.S., Fanestil, D.D. 1980. Covalent modification and inhibition of an epithelial sodium channel by tyrosine-reactive reagents.Am. J. Physiol. 239:F299-F306
Park, C.S., Kipnowski, J., Fanestil, D.D. 1983. Role of carboxyl group in Na+-entry step at apical membrane of toad urinary bladder.Am. J. Physiol. 245:F707-F715
Schultz, S.G. 1981. Homocellular regulatory mechanisms in sodium-transporting epithelia: Avoidance of extinction by “flush-through.”Am. J. Physiol. 241:F579-F590
Smith, R.L., Cochran, D.W., Cragoe, E.J., Gund, P. 1979. Studies on the tauterism and conformation of amiloride.In: Amiloride and Epithelial Sodium Transport. A.W. Cuthbert, G.M. Fanelli and A. Scriabine, editors. pp. 21–34. Urban and Schwarzenberg, Baltimore, Md.
Willis, N.K. 1981. Antibiotics as tools for studying the electrical properties of tight epithelia.Fed. Proc. 40:2202–2205
Wolfenden, R. 1983. Waterlogged molecules.Science 222:1087–1093
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garvin, J.L., Simon, S.A., Cragoe, E.J. et al. Phenamil: An irreversible inhibitor of sodium channels in the toad urinary bladder. J. Membrain Biol. 87, 45–54 (1985). https://doi.org/10.1007/BF01870698
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01870698