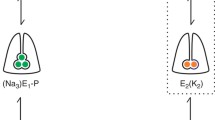Summary
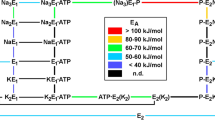
In the first part of the paper, evidence has been presented that electrochromic styryl dyes, such as RH 421, incorporate into Na, K-ATPase membranes isolated from mammalian kidney and respond to changes of local electric field strength. In this second part of the paper, fluorescence studies with RH-421-labeled membranes are described, which were carried out to obtain information on the nature of charge-translocating reaction steps in the pumping cycle. Experiments with normal and chymotrypsin-modified membranes show that phosphorylation by ATP and occlusion of Na+ are electroneutral steps, and that release of Na+ from the occluded state to the extracellular side is associated with translocation of charge. Fluorescence signals observed in the presence of K+ indicate that binding and occlusion of K+ at the extracellular face of the pump is another major electrogenic reaction step. The finding that the fluorescence signals are insensitive to changes of ionic strength leads to the conclusion that the binding pocket accommodating Na+ or K+ is buried in the membrane dielectric. This corresponds to the notion that the binding sites are connected with the extracellular medium by a narrow access channel (“ion well”). This notion is further supported by experiments with lipophilic ions, such as tetraphenylphosphonium (TPP+) or tetraphenylborate (TPB−), which are known to bind to lipid bilayers and to change the electrostatic potential inside the membrane. Addition of TPP+ leads to a decrease of binding affinity for Na+ and K+, which is thought to result from the TPP−-induced change of electric field strength in the access channel.
Similar content being viewed by others
References
Andersen, O.S., Feldberg, S., Nakadomari, H., Levy, S. McLaughlin, S. 1978. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes.Biophys. J. 21:35–70
Apell, H.-J. 1989. Electrogenic properties of the Na,K pump.J. Membrane Biol. 110:103–114
Apell, H.-J., Borlinghaus, R., Läuger, P. 1987. Fast charge translocations associated with partial reactions of the Na,K pump: II. Microscopic analysis of transient currents.J. Membrane Biol. 97:179–191
Apell, H.-J., Häring, V., Roudna, M. 1990. Na, K-ATPase in artificial lipid vesicles: Comparison of Na,K and Na-only pumping mode.Biochim. Biophys. Acta 1023:81–90
Bahinski, A., Nakao, M., Gadsby, D.C. 1988. Potassium translocation by the Na/K pump is voltage insensitive.Proc. Natl. Acad. Sci. USA 85:3412–3416
Beaugé, L., Berberian, G., Campos, M., Pedemonte, C. 1985. Substrate role of acetyl phosphate on Na,K-ATPase.In: The Sodium Pump. I. Glynn and C. Ellory, editors, pp. 321–333. Company of Biologists, Cambridge
Borlinghaus, R., Apell, H.-J., Läuger, P. 1987. Fast charge translocations associated with partial reactions of the Na,K-pump: I. Current and voltage transients after photochemical release of ATP.J. Membrane Biol. 97:161–178
Bühler, R., Stürmer, W., Apell, H.-J., Läuger, P. 1991. Charge translocation by the Na,K-pump: I. Kineticts of local field changes studies by time-resolved fluorescence measurement.J. Membrane Biol. 121:141–161
Cornelius, F. 1989. Uncoupled Na+-efflux on reconstituted shark Na,K-ATPase is electrogenic.Biochem. Biophys. Res. Commun. 160:801–807
Deguchi, N., Jørgensen, P.L., Maunsbach, A.B. 1977. Ultrastructure of the sodium pump. Comparison of thin sectioning, negative staining, and freeze-fracture of purified, membranebound (Na+, K+)-ATPase.J. Cell Biol. 75:619–634
De Weer, P., Gadsby, D.C., Rakowski, R.F. 1988. Voltage dependence of the Na−K pump.Annu. Rev. Physiol. 50:225–241
Fendler, K., Grell, E., Haubs, M., Bamberg, E. 1985. Pump currents generated by the purified Na+, K+-ATPase from kidney on black lipid membranes.EMBO J. 4:3079–3085
Forbush, B., III. 1987. Rapid release of42K and86Rb from two distinct transport sites on the Na,K-pump in the presence of Pi or vanadate.J. Biol. Chem. 262:11116–11127
Forbush, B., III 1988. Occluded ions and Na,K-ATPase.In: Na+, K+-Pump. Part A: Molecular Aspects. J.C. Skou, J.G. Nørby, A.B. Maunsbach, and M. Esmann, editors. pp. 229–248. A.R. Liss, New York
Glynn, I.M. 1985. The Na+, K+-transporting adenosine triphosphatase.In: The Enzymes of Biological Membranes. (2nd ed.) Vol. 3, pp. 35–114. A.N. Martonosi, editor. Plenum, New York
Glynn, I.M., Hara, Y., Richards, D.E. 1984. The occlusion of sodium ions within the manmalian sodium-potassium pump: Its role in sodium transport.J. Physiol. 351:531–547
Goldshleger, R., Shahak, Y., Karlish, S.J.D. 1990. Electrogenic and electroneutral transport modes of renal Na/K ATPase reconstituted into proteoliposomes.J. Membrane Biol. 113:139–154
Goldshlegger, R., Karlish, S.J.D., Rephaeli, A., Stein, W.D. 1987. The effect of membrane potential on the mammalian sodium-potassium pump reconstituted into phospholipid vesicles.J. Physiol. 387:331–355
Jørgensen, P.L., 1974. Isolation of the (Na−+K+)-ATPase.Methods Enzymol. 32:277–290
Jørgensen, P.L., Andersen, J.P. 1988. Structural basis for E1–E2 conformational transitions in Na,K-pump and Ca-pump proteins.J. Membrane Biol. 103:95–120
Jørgensen, P.L., Collins, J.H. 1986. Tryptic and chymotryptic cleavage sites in the sequence of α-subunit of (Na++K+)-ATPase from outer medulla of mammalian kidney.Biochim. Biophys. Acta 860:570–576
Jørgensen, P.L., Petersen, J. 1985. Chymotryptic cleavage of α-subunit in E1-forms of renal (Na++K+)-ATPase: Effects on enzymatic properties, ligand binding and cation exchange.Biochim. Biophys. Acta 821:319–333
Kapakos, J.G., Steinberg, M. 1986. Ligand binding to (Na,K)-ATPase labeled with 5-iodoacetamidofluorescein.J. Biol. Chem. 261:2084–2089
Kaplan, J.H., Forbush, B., III, Hoffman, J.F. 1978. Rapid photolytic release of adenosine-5′-triphosphate from a protected analogue: Utilization by the Na:K pump of human red blood cell ghosts.Biochemistry 17:1929–1935
Klodos, I., Forbush, B., III. 1988. Rapid conformational changes of the Na/K pump revealed by a fluorescent dye. RH-160.J. Gen. Physiol. 92:46a (abstr.)
Läuger, P., Apell, H.-J., 1986. A microscopic model for the current-voltage behavior of the Na,K-pump.Eur. Biophys. J. 13:305–321
Läuger, P., Apell, H.-J. 1988. Transient behaviour of the Na,K-pump: Microscopic analysis of nonstationary ion-translocation.Biochim. Biophys. Acta 944:451–464
Mitchell, P., Moyle, J. 1974. The mechanism of proton translocation in reversible proton-translocating adenosine triphosphatases.Biochem. Soc. (Spec. Publ.)4:91–111
Nakao, M., Gadsby, D.C. 1986. Voltage dependence of Na translocation by the Na/K pump.Nature 323:628–630
Nakao, M., Gadsby, D.C. 1989. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular muocytes.J. Gen. Physiol. 94:539–565
Nørby, J.G. 1987. Na,K-ATPase: Structure and kinetics. Comparison with other ion transport systems.Chem. Scripta 27B:119–129
Nørby, J.G., Klodos, I. 1988. The phosphointermediates of Na,K-ATPase.In: The Na−,K+-Pump. Part A: Molecular Aspects. J.C. Skou, J.G. Nørby, A.B. Maunsbach, and M. Esmann, editors, pp. 249–270. A. R. Liss, New York
Rakowski, R.F., Paxson, C.L. 1988. Voltage dependence of Na/K pump current inXenopus oocytes.J. Membrane Biol. 106:173–182
Rakowski, R.F., Vasilets, L.A., LaTona, J., Schwarz, W. 1991 A negative slope in the current-voltage relationship of the Na+/K+ pump inXenopus oocytes produced by reduction of external [K+].J. Membrane Biol. 121:177–187
Rakowski, R.F., Vasilets, L.A., Schwarz, W. 1990. Conditions for a negative slope in the current-voltage relationship of the Na/K pump inXenopus oocytes.Biophys. J. 57:182a
Rephaeli, A., Richards, D., Karlish, S.J.D. 1986. Electrical potential accelerates the E1P(Na)-E2P conformational transition of (Na,K)ATPase is reconstituted vesicles.J. Biol Chem. 261:12,437–12,440
Robinson, J.D., Flashner, M.S. 1979. The (Na++K+)-activated ATPase. Enzymatic and transport properties.Biochim. Biophys. Acta 549:145–176
Stürmer, W., Apell, H.-J., Wuddel, I., Läuger, P. 1989. Conformational transitions and charge translocation by the Na,K pump: Comparison of optical and electrical transients elicited by ATP-concentration jumps.J. Membrane Biol. 110:67–86
Tanford, C. 1983. Mechanism of free energy coupling in active transport.Annu. Rev. Biochem. 52:379–409
Yoda, A., Yoda, S. 1987. Two different phosphorylation-dephosphorylation cycles of Na,K-ATPase proteoliposomes accompanying Na+ transport in the absence of K+.J. Biol. Chem. 262:110–115
Author information
Authors and Affiliations
Additional information
Deceased (September 13, 1990).
Rights and permissions
About this article
Cite this article
Stürmer, W., Bühler, R., Apell, H.J. et al. Charge translocation by the Na,K-pump: II. Ion binding and release at the extracellular face. J. Membrain Biol. 121, 163–176 (1991). https://doi.org/10.1007/BF01870530
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870530