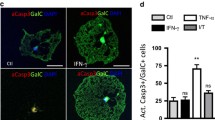
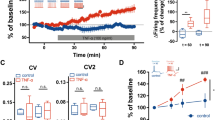
Summary
The effects of tumor necrosis factor-α (TNF-α), a cytokine secreted by activated macrophages, on the electrical membrane properties of cultured adult ovine oligodendrocytes (OLGs) were investigated using the whole-cell voltage-clamp technique. Treatment with recombinant human TNF-α (rhTNF) for 24 to 72 hr produces (i) process retraction in some but not all OLGs, (ii) a reduction in the resting membrane potential with no significant change in membrane capacitance or input resistance over control cells and (iii) a decrease in the expression of both the inwardly rectifying and outward K+ current. The magnitude of the membrane potential change as well as K+ current inhibition was larger in cells with retracted processes. The electrophysiological effects of rhTNF were attenuated when rhTNF was neutralized with a polyclonal anti-rhTNF antibody. The binding of rhTNF to its receptor has been reported to increase GTP binding, to increase GTPase activity of a pertussis-sensitive G protein, and to produce an elevation in intracellular cAMP in other cell types. However, pretreatment of OLGs with activated pertussis toxin failed to attenuate or mimic the effects of rhTNF. Chronic exposure of OLGs to the membrane permeant analogue of cAMP, 8-bromo-cAMP, resulted primarily in an inhibition of the inwardly rectifying K+ current, an effect which was less than that produced by rhTNF alone and without any of the associated rhTNF-induced morphological changes. This indicates that the effects of rhTNF cannot be entirely accounted for by an elevation in intracellular cAMP. Cycloheximide (CHX), an inhibitor of protein synthesis, mimicked the effects of rhTNF; however, the effects of rhTNF and CHX were not additive. The finding that both ionic current expression and membrane potential were reduced in cells treated with rhTNF that appeared morphologically normal suggests that abnormal ion channel expression in OLGs precedes and may contribute to eventual myelin swelling and damage.
Similar content being viewed by others
References
Barres, B.A., Chun, L.L.Y., Corey, D.P. 1989. A calcium current in cortical astrocytes: Induction by cAMP and neurotransmitters, and permissive effect of serum factors.J. Neurosci. 9:3169–3175
Barres, B.A., Chun, L.L.Y., Corey, D.P. 1990. Ion channels in vertebrate glia.Annu. Rev. Neurosci. 13:441–474
Benveniste, E.N., Merill, J.E. 1986. Stimulation of oligodendrog-lial proliferation and maturation by interleukin-2.Nature 321:610–613
Bernton, E.W., Beach, J.E., Holaday, J.W., Smallridge, R.C., Fein, H.G. 1987. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells.Science 238:519–522
Caffrey, J.M., Brown, A.M., Schneider, M.D. 1989. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: Reversible suppression by transforming growth factor beta-1, an inhibitor of myogenic pathway.J. Neurosci. 9:3443–3453
Cullen, M.J., Webster, H. deF. 1989. Inhibition of protein synthesis during CNS myelination produces focal accumulations of membrane vesicles in oligodendrocytes.J. Neurocytol. 18:763–774
Favaron, M., Manev, H., Alho, H., Bertolino, M., Ferret, B., Guidotti, A., Costa, E. 1988. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex.Proc. Nat'l Acad. Sci. USA 85:7351–7355
Giulian, D., Vaca, K., Johnson, B. 1988. Secreted peptides as regulators of neuron-glia and glia-glia interactions in the developing nervous system.J. Neurosci. Res. 21:487–500
Granger, G.A., Knauer, M.F., Fitzgerald, T.P., Yamamoto, R.S., Longmuir, K.J. 1990. Phospholipase activation in murine L929 cells during in vitro destruction by recombinant human TNF and lymphotoxin.In: Tumor Necrosis Factor: Structure, Mechanism of Action, Role in Disease and Therapy, B. Bonavida and G. Granger, editors. pp. 67–69. Karger, Basel
Heino, J., Ignotz, R.A., Hemler, M.E., Crouse, C., Massague, J. 1989. Regulation of cell adhesion receptors by transforming growth factor-β.J. Biol. Chem. 264:380–388
Hensel, G., Mannel, D., Pfizenmaier, K., Kronke, M. 1987. Autocrine stimulation of TNF-α mRNA expression in HL-60 cells.Lymphokine Res. 6:119–125
Hertz, L., Soliven, B., Hertz, E., Szuchet, S., Nelson, D.J. 1990. Channel-mediated and carrier-mediated uptake of K+ into cultured ovine oligodendrocytes.Glia 3:550–557
Hori, T., Shibata, M., Nakashima, T., Yamasaki, M., Asami, A., Asami, T., Koga, H. 1988. Effects of interleukin-1 and arachidonate on the preoptic and anterior hypothalamic neurons.Brain Res. Bull. 20:75–82
Howe, J.R., Ritchie, J.M. 1990. Sodium currents in Schwann cells from myelinated and nonmyelinated nerves of neonatal and adult rabbits.J. Physiol. 425:169–210
Imamura, K., Sherman, M.L., Spriggs, D., Kufe, D. 1988. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells.J. Biol. Chem. 263:10247–10253
Lavi, E., Suzumura, A., Murasko, D.M., Murray, E.M., Silberberg, D.H., Weiss, S.R. 1988. Tumor necrosis factor induces the expression of MHC class I antigens on mouse astrocytes.Ann. N.Y. Acad. Sci. 540:488–490
McPherson, G.A. 1983. A practical computer-based approach to the analysis of radioligand binding experiments.Comput. Programs Biomed. 17:107–113
Old, L.J. 1990. Tumor necrosis factor.In: Tumor Necrosis Factor: Structure Mechanism of Action, Role in Disease and Therapy, B. Bonavida and G. Granger, editors. pp. 1–30. Karger, Basel
Pohlman, T.H., Harlan, J.M. 1989. Human endothelial cell response to lipopolysaccharide, interleukin-1 and tumor necrosis factor is regulated by protein synthesis.Cell. Immunol. 119:41–52
Robbins, D.S., Shirazi, Y., Drysdale, B., Lieberman, A., Shin, H.S., Shin, M.L. 1987. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes.J. Immunol. 139:2593–2597
Saneto, R.P., Altman, A.A., Knobler, R.L., Johnson, H.M., de Vellis, J. 1986. Interleukin-2 mediates the inhibition of oligodendrocyte progenitor cell proliferationin vitro.Proc. Natl's Acad. Sci. USA 83:9221–9226
Sapolsky, R., Rivier, C., Yamamoto, G., Plotsky, P., Vale, W. 1987. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor.Science 238:522–524
Scheurich, P., Ucer, U., Kronke, M., Pfizenmaier, K. 1986. Quantitation and characterization of high affinity membrane receptors for tumor necrosis factor on human leukemic cell lines.Int. J. Cancer 38:127–133
Schutze, S., Scheurich, P., Pfizenmaier, K., Kronke, M. 1989. Tumor necrosis factor signal transduction.J. Biol. Chem. 264:3562–3567
Selmaj, K.W., Farooq, M., Norton, T., Raine, C.S., Brosnan, C.F. 1990. Proliferation of astrocytes in vitro in response to cytokines.J. Immunol. 144:129–135
Selmaj, K.W., Raine, C.S. 1988. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro.Ann. Neurol. 23:339–346
Soliven, B., Szuchet, S., Arnason, B.G.W., Nelson, D.J. 1988a Voltage-gated potassium currents in cultured ovine oligodendrocytes.J. Neurosci. 8:2131–2141
Soliven, B., Szuchet, S., Arnason, B.G.W., Nelson, D.J. 1988b. Forskolin and phorbol esters decrease the same K+ conductance in cultured oligodendrocytes.J. Membrane Biol. 105:177–186
Stauber, G.B., Aiyer, R.A., Aggarwal, B.B. 1988. Human tumor necrosis factor-α receptor.J. Biol. Chem. 263:19098–19104
Suzumura, A., Silberberg, D.H. 1989. Lymphokines facilitate maturation of oligodendrocytesin vitro.Brain. Res. 480:51–57
Szuchet, S., Arnason, B.G.W., Polak, P.E. 1980. Separation of ovine oligodendrocytes into two distinct bands on a linear sucrose gradient.J. Neurosci. Methods. 3:7–19
Szuchet, S., Polak, P.E., Yim, S.H. 1986. Mature oligodendrocytes cultured in the absence of neurons recapitulate the ontogenic development of myelin membranes.Dev. Neurosci. 8:208–221
Szuchet, S., Yim, S.H. 1984. Characterization of a subset of oligodendrocytes separated on the basis of selective adherence properties.J. Neurosci. Res. 11:131–144
Szuchet, S., Yim, S.H., Monsma, S. 1983. Lipid metabolism of isolated oligodendrocytes maintained in long term culture mimics events associated with myelinogenesis.Proc. Nat'l Acad. Sci. USA 80:7019–7023
Tracey, K.J., Lowry, S.F., Beutler, B., Cerami, A., Albert, J.D., Shires, G.T. 1986. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential.J. Exp. Med. 164:1368–1373
Tsujimoto, M., Yip, Y.K., Vilcek, J. 1985. Tumor necrosis factor: Specific binding and internalization in sensitive and resistant cells.Proc. Nat'l Acad. Sci. USA 82:7626–7630
Vartanian, T., Dawson, G., Soliven, B., Nelson, D.J., Szuchet, S. 1989. Phosphorylation of myelin basic protein in intact oligodendrocytes: Inhibition by galactosylsphingosine and cyclic AMP.Glia 2:370–379
Vartanian, T., Szuchet, S., Dawson, G., Campagnoni, A.T. 1986. Oligodendrocyte adhesion activates protein kinase C-mediated phosphorylation of myelin basic protein.Science 234:1395–1398
Wong, G.H., Bartlett, P.F., Clark-Lewis, I., Battye, F., Schrader, J.W. 1984. Inducible expression of H-2 and Ia antigens on brain cells.Nature 310:688–691
Wilson, R., Brophy, P.J. 1989. Role for the oligodendrocyte cytoskeleton in myelination.J. Neurosci. Res. 22:439–448
Yim, S.H., Szuchet, S., Polak, P.E. 1986. Cultured oligodendrocytes. A role for cell-substratum interaction in phenotypic expression.J. Biol. Chem. 261:11808–11815
Zhang, Y., Lin, J., Yip, Y.K., Vilcek, J. 1988. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: Role in the induction of interleukin 6.Proc. Nat'l Acad. Sci. USA 85:6802–6805
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soliven, B., Szuchet, S. & Nelson, D.J. Tumor necrosis factor inhibits K+ current expression in cultured oligodendrocytes. J. Membrain Biol. 124, 127–137 (1991). https://doi.org/10.1007/BF01870457
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870457