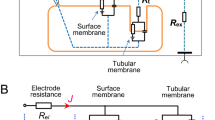
Summary
Mammalian urinary bladder undergoes, in a 24-hour period, a series of slow fillings and rapid emptying. In part the bladder epithelium accommodates volume increase by stretching the cells so as to eliminate microscopic folds. In this paper we present evidence that once the cells have achieved a smooth apical surface, further cell stretching causes an insertion of cytoplasmic vesicles resulting in an even greater apical surface area per cell and an enhanced storage capacity for the bladder. Vesicle insertion was stimulated by application of a hydrostatic pressure gradient which caused the epithelium to bow into the serosal solution. Using capacitance as a direct and nondestructive measure of area we found that stretching caused a 22% increase in area. Removal of the stretch caused area to return to within 8% of control. An alternate method for vesicle insertion was swelling the cells by reducing mucosal and serosal osmolarity. This perturbation resulted in a 74% increase in area over a 70-min period. Returning to control solutions caused area to decrease as a single exponential with an 11-min time constant. A microtubule blocking agent (colchicine) dit not inhibit the capacitance increase induced by hypoosmotic solutions, but did cause an increase in capacitance in the absence of a decreased osmolarity. Microfilament disrupting agent (cytochalasin B, C, B.) inhibited any significant change in capacitance after osmotic challenge. Treatment of bladders during swelling with C.B. and subsequent return, to control solutions increased the time constant of the recovery to control values (22 min). The Na+-transporting ability of the vesicles was determined and found to be greater than that of the apical membrane. Aldosterone increased the transport ability of the vesicles. We conclude that some constituent of urine causes a loss of apical membrane permeability. Using electrophysiological methods we estimated that the area of cytoplasmic vesicles is some 3.3 times that of the apical membrane area. We discuss these results in a general model for vesicle translocation in mammalian urinary bladder.
Similar content being viewed by others
References
Arruda, J.A., Sabatini, S., Mola, R., Dytko, G. 1980. Inhibition of H+ secretion in the turtle bladder by colchicine and vinblastine.J. Lab. Clin. Med. 96:450–459
Benos, D.J., Mandel, L.J., Simon, S.A. 1980. Effects of chemical group specific reagents on sodium entry and the amiloride binding site in frog skin: Evidence for separate sites.J. Membrane Biol. 56:149–158
Clausen, C., Lewis, S.A., Diamond, J.M. 1979. Impedance analysis of a tight epithelium using a distributed resistance model.Biophys. J. 26:291–318
Cole, K.S. 1972. Membranes, Ions and Impulses. University of California Press, Berkeley
Diamond, J.M., Machen, T.E. 1983. Impedance analysis in epithelia and the problem of gastric acid secretion.J. Membrane Biol. 72:17–41
Dick, H.J., Lindemann, B. 1975. Saturation of Na-current in frog skin epithelium abolished by PCMB.Pfluegers Arch. 355:R72
Gluck, S., Cannon, C., Al-Awqati, Q. 1982. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane.Proc. Natl. Acad. Sci. USA 79:4327–4331
Gottlieb, G.P., Turnheim, K., Frizzel, R.A., Schultz, S.G. 1978.p-Chloromercuribenzinesulfonate blocks and reverses the effect of amiloride on sodium transport across rabbit colonin vitro.Biophys. J. 22:125–129
Hegel, U. 1977. AC analysis of transporting epithelia.In: Diuretics in Research and Clinics. W. Siegenthaler, R. Beckerhoff, and W. Vether, editors. pp. 21–29. Georg Thieme, Stuttgart
Hicks, R.M. 1965. The fine structure of the transitional epithelium of rat ureter.J. Cell. Biol. 26:25–48
Kasbekar, D.K., Obrink, K.J., Flemstrom, G. 1978. Microtubule disrupting agents and gastric secretion.Proc. Symp. Gastric. Ion Transport. Acta Physiol. Scand. Special Suppl. pp. 253–266 Uppsala, Sweden
Koss, L.G. 1969. The asymmetric unit membrane of the epithelium of the urinary bladder of the rat. An electron microscopic study of a mechanism of epithelial maturation and function.Lab. Invest. 21:154–168
Leeson, R. 1962. Histology, histochemistry and electron microscopy of the transitional epithelium of the rat urinary bladder in response to induced physiological changes.Acta Anat. 48:297–315
Lewis, S.A. 1977. A reinvestigation of the function of the mammalian urinary bladder.Am. J. Physiol. 232:F187-F195
Lewis, S.A., Clausen, R., Diamond, J.M. 1976.Appendix In: Na+ transport by rabbit urinary bladder, a tight epithelium.J. Membrane Biol. 28:35–40
Lewis, S.A., Diamond, J.M. 1976. Na+ transport by rabbit urinary bladder, a tight epithelium.J. Membrane Biol. 28:1–40
Lewis, S.A., Wills, N.K. 1983. Apical membrane permeability and kinetic properties of the sodium pump in rabbit urinary bladder.J. Physiol. (London) 341:169–184
Loo, D.D.F., Lewis, S.A., Ifshin, M.S., Diamond, J.M. 1983. Turnover, membrane insertion, and degradation of sodium channels in rabbit urinary bladder.Science 221:1288–1290
Minsky, B.D., Chlapowski, F.J. 1978. Morphometric analysis of the translocation of luminal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder.J. Cell Biol. 77:685–697
Porter, K.R., Kenyon, K., Badenhausen, S. 1965. Origin of discoidal vesicles in cells of the transitional epithelium.Anat. Rec. 151:401a
Porter, K.R., Kenyon, K., Badenhausen, S. 1967. Specializations of the unit membrane.Protoplasma 63:262–274
Richter, W.R., Moize, S.M. 1963. Electron microscopic observations on the collapsed and distended mammalian urinary bladder.J. Ultrastruct. Res. 9:1–9
Schwartz, G.J., Burg, M.B. 1978. Mineralocorticoid effects on cation transport by cortical collecting tubulesin vitro.Am. J. Physiol. 235:F576-F585
Staehelin, L.A., Chlapowski, F.J., Bonneville, M.A. 1972. Luminal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images.J. Cell Biol. 53:73–91
Stetson, D.L., Lewis, S.A., Alles, W., Wade, J.B. 1982. Evaluation by capacitance measurements of antidiuretic hormone induced membrane area changes in toad bladder.Biochim. Biophys. Acta 689:267–274
Suzuki, K., Kottra, G., Kampmann, L., Fromter, E. 1982. Square wave pulse analysis of cellular and paracellular conductance pathways inNecturus gallbladder epithelium.Pfluegers Arch. 394:302–312
Taylor, A., Mamelak, M., Reaven, E., Maffly, R. 1973. Vasopressin: Possible role of microtubules and microfilaments in its action.Science 181:347–350
Wade, J.B., Stetson, D.L., Lewis, S.A. 1981. ADH action: Evidence for a membrane shuttle mechanism.Ann. N.Y. Acad. Sci. 372:106–117
Walker, B.E. 1960. Electron microscopic observations on transitional epithelium of mouse urinary bladder.J. Ultrastruct. Res. 3:345–361
Warncke, J., Lindemann, B. 1981. Effect of ADH on the capacitance of apical epithelial membranes.Proc. Int. Union. Phys. Sci. Vol. 3, pp. 129–133. Pergamon, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lewis, S.A., de Moura, J.L.C. Apical membrane area of rabbit urinary bladder increases by fusion of intracellular vesicles: An electrophysiological study. J. Membrain Biol. 82, 123–136 (1984). https://doi.org/10.1007/BF01868937
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01868937