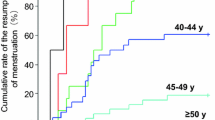
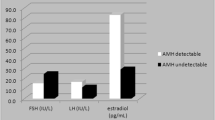
Summary
Cyclophosphamide and other alkylating agents suppress ovarian function in pre-menopausal women. However, endocrine details remain unknown regarding the influence of patients' age and obesity on CMF-induced hormonal changes. We studied changes in endocrine profile due to chemotherapy (CMF) in 70 pre-menopausal patients with axillary node positive, stage II and/or III breast carcinoma. Plasma levels of estrone (E1), estradiol (E2), androstenedione (A2), luteinizing hormone (LH), and prolactin (PRL) were determined on day 1 and 8 of each chemocycle for 12 cycles. After receiving therapy, 23% of the women continued to have regular menstrual cycles (non-amenorrheic group). In the remaining 77%, ovarian function was suppressed, as evidenced by the onset of amenorrhea within 0–11 months (amenorrheic group). The mean time to amenorrhea was 2.83±0.33 months (SE). The time required to develop amenorrhea inversely correlated to the patient's age. Both incidence of amenorrhea and time to amenorrhea remained unaffected by either patients' obesity or the timing of chemotherapy initiation in relation to menstrual cycle phase (progestational, follicular). Plasma hormone levels fluctuated widely in both groups during the first three chemocycles. During chemocycle months 4 to 10, in the amenorrheic group, plasma E1, E2, and P declined to their baseline levels with a concomitant rise in LH levels. At this time, E1, E2, and P levels were significantly lower in amenorrheics, despite menstrual cycle associated fluctuations in the non-amenorrheic group. Estrogens (E1 and E2) gradually declined further following the onset of amenorrhea in subsequent months. Further data analysis suggests that host age or obesity did not influence CMF-induced changes in the plasma endocrine profile.
Similar content being viewed by others
References
Bonadonna G, Rossi A, Valagussa A, Banif A, Veronesi U: The CMF program for operable breast cancer with positive axillary nodes. Cancer 39: 2904–2915, 1977
Bonadonna G, Brusamolino E, Valagussa P, Rossi A,et al.: Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 294: 405–410, 1976
Hsu AC, Folami AO, Bain J, Rauce CP: Gonadal function in males treated with cyclophosphamide for nephrotic syndrome. Fertil Steril 31: 173–177, 1979
Chapman RM, Sutcliffe SB, Malpas JP: Cytotoxic induced ovarian failure in women with Hodgkin's disease. JAMA 242: 1877–1881, 1979
Rose DP, Davis TE: Ovarian function in patients receiving adjuvant chemotherapy for breast cancer. Lancet i: 1174–1176, 1977
Rose DP, Davis TE: Effects of adjuvant chemohormonal therapy on the ovarian and adrenal function of breast cancer patients. Cancer Res 40: 4043–4047, 1980
Costagnetta L, Traina A, Ciaccio M, Carruba U, Polito L, DiCarlo A: Modulation of oestrogen, excretion profiles by adjuvant chemotherapy in pre- and post-menopausal breast cancer. J Steroid Biochem 23: 1115–1122, 1985
Shamberger RC, Sherins RJ, Zieglert L, Glatstein E, Rosenberg SA: Effects of postoperative adjuvant chemotherapy and radiotherapy on ovarian function in women undergoing treatment for soft tissue sarcoma. J Natl Cancer Inst 67: 1213–1218, 1981
Dnistrian AM, Schwartz MK, Fracchia AA, Kaufman RJ, Makes TB, Currie VE: Endocrine consequences of CMF adjuvant therapy in pre-menopausal and post-menopausal breast cancer patients. Cancer 51: 803–807, 1983
Wright K, Collins DC, Preedy JRK: The use of specific radioimmunoassays to determine the renal clearance rates of estrone and estradiol during the menstrual cycle. J Clin Endocrinol Metab 47: 1084–1091, 1978
Longcope C, Franz C, Morello C, Baker R, Johnston CJ: Steroid and gonadotrophin levels in women during the perimenopausal years. Maturitas 8: 189–196, 1986
Vermeulen A, Lerdonck L: Sex hormone concentrations in post-menopausal women. Clin Endocrinol 9: 59–66, 1978
Vermeulen A: Sex hormone status of the post-menopausal women. Maturitas 2: 81–89, 1980
Siiteri PK, McDonald PC: Role of extraglandular estrogen in human endocrinology. In: Greep RO, Astwood EB (eds)Handbook of Physiology 7, Vol II, Part I. Physiological Society, Washington, 1973, pp 615–628
Longcope C: Steroid production in pre and post-menopausal women. In: Greenblatt RB, Mahesh VB, McDonald PC (Eds)Menopausal syndrome. Williams and Wilkins, Baltimore, 1974, p 6.
Poortman J, Thijssen JHH, Dewaard F: Plasma estrone, estradiol and androstenedione levels in post-menopausal women: Relation to body weight and height. Maturitas 3: 65–71, 1981
Meldrum DR, Davidson BJ, Tataryn IV, Judd HL: Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol 57: 624–628, 1981
McDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK: Effect of obesity on conversion of plasma androstenedione to estrone in post-menopausal women with and without endometrial cancer. Am J Obstet-Gynecol 130: 448, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehta, R.R., Beattie, C.W. & Das Gupta, T.K. Endocrine profile in breast cancer patients receiving chemotherapy. Breast Cancer Res Tr 20, 125–132 (1991). https://doi.org/10.1007/BF01834642
Issue Date:
DOI: https://doi.org/10.1007/BF01834642