Abstract
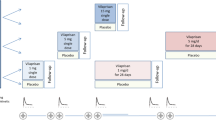
A Phase I study of the new antiestrogenic drug toremifene was carried out in 27 Japanese women, using oral doses of 10–480 mg per day for one or five days. Serum concentrations reached a dose-dependent maximum 2–6 hours after each oral administration. Side effects were generally mild.
Similar content being viewed by others
References
Kangas L, Nieminen A, Blanco G,et al.: A new triphenylethylene compound, FC-1157a. II. Antitumor effects. Cancer Chemother Pharmacol 17: 109–113, 1986
Robinson SP, Mauel DA, Jordan VC: Antitumor actions of toremifene in the 7,12-dimethylbenzanthracene (DMBA)-induced rat mammary tumor model. Eur J Cancer Clin Oncol 24: 1817–1821, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tominaga, T., Abe, O., Izuo, M. et al. A phase I study of toremifene. Breast Cancer Res Tr 16 (Suppl 1), S (1990). https://doi.org/10.1007/BF01807141
Issue Date:
DOI: https://doi.org/10.1007/BF01807141