Summary
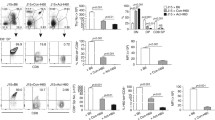
We have previously shown that, as a consequence of low-dose melphalan (l-phenylalanine mustard) treatment, thymocytes from mice bearing a large, day-10 MOPC-315 tumor, but not thymocytes from normal mice, acquire the ability to generate an enhanced level of antitumor cytotoxicity upon in vitro stimulation with MOPC-315 tumor cells plus low concentrations (9.0–90 IU/ml) of exogenous interleukin-2 (IL-2) [23]. Here we show that the time interval between tumor inoculation and low-dose melphalan therapy as well as the magnitude of tumor burden at the time of the chemotherapy are important for the ability of the drug to render thymocytes more responsive to in vitro stimulation with MOPC-315 tumor cells plus low concentrations of recombinant IL-2 (rIL-2). Specifically, the chemotherapy was found to be effective in enhancing the thymic antitumor reactivity only if the mice bore a large, late-stage tumor. Comparison of thymocytes from untreated mice bearing a large, late-stage tumor to thymocytes from normal mice revealed that tumor-bearer thymocytes contained approximately a three-fold higher frequency of cytotoxic T lymphocyte precursors (CTLp) for MOPC-315-associated antigens. Following curative low-dose melphalan therapy of mice bearing a large, latestage MOPC-315 tumor, the frequency of CTLp for MOPC-315-associated antigens increased further, reaching a level approximately tenfold higher than that found among thymocytes of normal mice. At the same time, the frequency of CTLp for an antigenically unrelated allogeneic tumor (EL4) as well as the overall percentage of mature cells was not increased. The cells responsible for the exertion of the enhanced antitumor cytotoxicity following in vitro stimulation of thymocytes from mice treated with low-dose melphalan when they have a large, latestage MOPC-315 tumor are of the CD8+/CD4− phenotype. Thus, the enhanced level of antitumor cytotoxicity generated by thymocytes from mice that are treated with low-dose melphalan when they have a large, late-stage MOPC-315 tumor is due, at least in part, to the presence of an enlarged pool of CTLp specific for MOPC-315-associated antigens, which mature into CD8+/CD4− effector cells upon stimulation with MOPC-315 tumor cells plus low concentrations of rIL-2.
Similar content being viewed by others
References
Barker E, Mokyr MB (1988) Importance of Lyt 2+ T-cells in the resistance of melphalan cured MOPC-315 tumor bearers to a challenge with MOPC-315 tumor cells. Cancer Res 48: 4834
Bartik MM, Takesue BY, Mokyr MB (1987) Melphalan-induced enhancement of antitumor immune reactivity in thymocytes of adult BALB/c mice bearing a large MOPC-315 tumor. Cancer Res 47: 4848
Ben-Efraim S, Bocian RC, Mokyr MB, Dray S (1985) Increase in the effectiveness of melphalan therapy with progression of MOPC-315 plasmacytoma tumor growth. Cancer Immunol Immunother 15: 101
Bocian RC, Ben-Efraim S, Dray S, Mokyr MB (1984) Melphalan-mediated potentiation of antitumor immune responsiveness of immunosuppressed spleen cells from mice bearing a large MOPC-315 tumor. Cancer Immunol Immunother 18: 41
Burton RC (1986) Frequency of murine cytotoxic T cell precursors reactive to plasma cell tumour-associated antigens as compared to major histocompatibility antigens. Aust J Exp Med Biol Sci 64: 323
Chassoux DM, Gotch FM, MacLennan ICM (1978) Analysis of synergy between cyclophosphamide therapy and immunity against a mouse tumour. Br J Cancer 38: 211
Fink PJ, Bevan MJ, Weissman IL (1984) Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J Exp Med 159: 436
Glaser M (1979) Regulation of specific cell-mediated cytotoxic response against SV40-induced tumor associated antigens by depletion of suppressor T cells with cyclophosphamide in mice. J Exp Med 149: 774
Hancock EJ, Kilburn DG, Levy JG (1981) Helper cells active in the generation of cytotoxicity to a syngeneic tumor. J Immunol 127: 1394
Hengst JCD, Mokyr MB, Dray S (1980) Importance of timing in cyclophosphamide therapy of MOPC-315 tumor bearing mice. Cancer Res 40: 2135
Hengst JCD, Mokyr MB, Dray S (1981) Cooperation between cyclophosphamide tumoricidal activity and host antitumor immunity in the cure of mice bearing large MOPC-315 tumors. Cancer Res 41: 2163
Hilgard P, Pohl J, Stekar J, Voegeli R (1985) Oxazaphosphorines as biological response modifiers: experimental and clinical perspectives. Cancer Treat Rev 12: 155
Ksander BR, Hendricks RL (1987) Cell-mediated immune tolerance to HSV-1 antigens associated with reduced susceptibility to HSV-1 corneal lesions. Invest Ophthalmol Vis Sci 28: 1986
Lefkovits I, Waldmann H (1984) Limiting dilution analysis of the cells of the immune system. I. The clonal basis of the immune response. Immunol Today 5: 265
Lewis GK, Kamin R (1980) Separation of T and B cells using plastic surfaces coated with anti-immunoglobulin antibodies (“panning”). In: BB Mishell and SM Shiigi (eds) Selected methods in cellular immunology. Freeman, San Francisco, pp 227–234
Lubet RA, Carlson DE (1978) Therapy of the murine plasmacytoma MOPC-104E: Role of the immune response. J Natl Cancer Inst 61: 897
Mathé G, Halle-Pannenko O, Bourut C (1977) Effectiveness of murine leukemia chemotherapy according to the immune state. Cancer Immunol Immunother 2: 139
Mathieson BJ, Fowlkes BJ (1984) Cell surface antigen expression on thymocytes: development and phenotypic differentiation of intrathymic subsets. Immunol Rev 82: 141
Mokyr MB (1987) Role of antitumor immunity in chemotherapy-induced tumor rejection. In: Den Otter W and Ruitenberg EJ (eds) Tumor immunology: mechanisms, diagnosis and therapy. Elsevier, Amsterdam, pp 309–325
Mokyr MB, Braun DP, Usher D, Reiter H, Dray S (1978) The development of in vitro and in vivo antitumor cytotoxicity in noncytotoxic MOPC-315 tumor bearer spleen cells “educated” in vitro with MOPC-315 tumor cells. Cancer Immunol Immunother 4: 143
Mokyr MB, Hengst JCD, Dray S (1982) Role of antitumor immunity in cyclophosphamide-induced rejection of subcutaneous nonpalpable MOPC-315 tumors. Cancer Res 42: 974
Mokyr MB, Barker E, Weiskirch LM, Takesue BY, Pyle JM (1989) Importance of Lyt 2+ T-cells in the curative effectiveness of a low dose of melphalan for mice bearing a large MOPC-315 tumor. Cancer Res 49: 4597
Mokyr MB, Bartik MM, Ahn M-C (1989) Interleukin 2 requirement for the in vitro generation of antitumor cytotoxicity by thymocytes from melphalan-cured MOPC-315 tumor bearers. Cancer Res 49: 870
Morikawa K, Hosokawa M, Hamada J, Sugawara M, Kobayashi H (1985) Hostmediated therapeutic effects produced by appropriately timed administration of bleomycin on a rat fibrosarcoma. Cancer Res 45: 1502
Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T (1988) IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol 141: 1543
Reisner Y, Linker-Israeli M, Sharon N (1976) Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol 25: 129
Rollinghoff M, Rouse BT, Warner NL (1973) Tumor immunity to murine plasma tumors. I. Tumor-associated transplantation antigens of NZB and BALB/c plasma cell tumors. J Natl Cancer Inst 50: 159
Santos GW, Owens AH, Sensenbrenner LL (1964) Effects of selected cytotoxic agents on antibody production in man, a preliminary report. Ann NY Acad Sci 114: 404
Scollay R, Shortman K (1985) Identification of early stages of T thymocyte development in the thymus cortex and medulla. J Immunol 134: 3632
Stutman O (1978) Intrathymic and extrathymic T cell maturation. Immunol Rev 42: 138
Takatsu K, Kikuchi Y, Kanatani T, Okuno K, Hamaoka T, Tominaga A, Sano Y (1986) Generation of cytotoxic T lymphocytes from thymocyte precursors to trinitrophenyl-modified self antigen. I. Requirement of both killer-helper factor(s) and interleukin 2 for CTL generation for a subpopulation of thymocytes. J Immunol 136: 1161
Takesue BY, Bartik MM, Mokyr MB (1987) Melphalan-induced appearance of potent antitumor reactivity in tumor bearer lymphocytes coexpressing the Lyt 2 and L3T4 antigens. Int J Immunopharmacol 9: 705
Weber E, Kees UR, Krammer PH (1987) Computer analysis of data from limiting dilution experiments of cells of the immune system. University of Heidelberg Press, Heidelberg FRG
Yamamura Y, Proctor J, Fischer BH, Harnaha JB, Mahvi TA (1980) Collaboration between specific antitumor immunity and chemotherapeutic agents. Int J Cancer 25: 417
Author information
Authors and Affiliations
Additional information
supported by Research Grant CA-35761 from the National Cancer Institute and Research Grant EY-05945 from the National Eye Institute. This work is in partial fulfillment of the requirements for the Doctor of Philosophy degree of M. M. B. M. B. M. was supported by a Career Development Award CA-01350 from the National Cancer Institute.
Rights and permissions
About this article
Cite this article
Bartik, M.M., Ahn, MC., Baumgartel, B.A. et al. Presence of an enlarged pool of MOPC-315-specific cytotoxic T lymphocyte precursors in the thymuses of mice that eradicated a large MOPC-315 tumor as a consequence of low-dose melphalan therapy. Cancer Immunol Immunother 32, 143–153 (1990). https://doi.org/10.1007/BF01771449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01771449