Summary
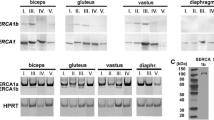
Using a monoclonal antibody (CDC4) that recognizes both the cardiac and slow skeletal isoforms of troponin T in an immunoblotting procedure, the composition of troponin T isoforms in adult and developing skeletal muscles of the rat and human were studied. Two major isoforms of slow troponin T (HS1 and HS2) were detected in all the adult human skeletal muscles investigated. Significant amounts of another isoform (HS3) in addition to HS1 and HS2 were also detectable in a subgroup of these muscles. All the human fetal skeletal muscles at 20 weeks of gestation expressed HS1 and HS2 isoforms but not HS3. The fetal skeletal muscles, however, also expressed cardiac troponin T in addition.
Unlike the human skeletal muscles, only a single isoform of slow troponin T was detected by antibody CDC4 in both the adult and neonatal rat skeletal muscles. The investigation of fetal rat skeletal muscles using the same antibody, however, detected the presence of not only the embryonic cardiac and adult slow skeletal isoforms but also five additional not previously described isoforms (Es1-Es5) of troponin T. These embryonic isoforms, Es1-Es5, were undetectable in the postnatal skeletal muscles although their small amounts could be detected in the neonatal rat hearts. The analysis of individual skeletal muscles from different parts of the body at different stages; of fetal development showed marked variations in both the composition of troponin T isoforms and the time sequence of their transitions in each muscle.
Similar content being viewed by others
References
Abe, H., Komiya T. &Obinata, T. (1986) Expression of multiple troponin T variants in neonatal chicken breast muscle.Dev. Biol. 118, 42–51.
Anderson, P. A. &Oakeley, A. E. (1989) Immunological identification of five troponin T isoforms reveals an elaborate maturational troponin T profile in rabbit myocardium.Circ. Res. 65, 1087–93.
Bird, I., Dhoot, G. K. &Wilkinson, J. M. (1985) Identification of multiple variants of fast muscle troponin T in the chicken using monoclonal antibodies.Eur. J. Biochem. 150, 517–25.
Breitbart, R. E., Nguyen, H. T., Medford, R. M., Destree, A. T., Mahadavi, V. &Nadal-Ginard, B. (1985) Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene.Cell 41, 67–82.
Bucher, E. A., Brousse, F. C. &Emerson, C. P. (1989) Developmental and muscle-specific regulation of avian fast skeletal troponin T isoform expression by mRNA splicing.J. Biol. Chem. 264, 12482–91.
Butler-Browne, G. S., Bugaisky, L. B., Cuenoud, S., Schwartz, K. &Whalen R. (1982) Denervation of newborn rat muscles does not block the appearance of adult fast myosin heavy chain.Nature 299 830–3.
Butler-Browne, G. S., Herlicoviez, D. &Whalen R. (1984) Effects of hypothyroidism on myosin isozyme transitions in developing rat muscle.FEBS Lett. 166, 71–5.
Cooper, T. A. &Ordahl, C. P. (1985) A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternative splicing.J. Biol. Chem. 260, 11140–8.
Dhoot, G. K. (1988) Identification and distribution of the fast class of troponin T in the adult and developing avian skeletal muscle.J. Muscle Res. & Cell Motil. 9, 446–55.
Dhoot, G. K. (1989) Evidence for the presence of a distinct embryonic isoform of myosin heavy chain in chicken skeletal muscle.Differentiation 40, 176–53.
Dhoot, G. K. (1990) Isoforms of troponin components in developing muscle fibres.The dynamic state of muscle fibres (edited by Pette, D. & Gruyter, W. de) Berlin, New York, pp. 165–179.
Dhoot, G. K., Frearson, N. &Perry, S. V. (1979) Polymorphic forms of troponin T and troponin C and their localization in striated muscle cell types.Exp. Cell Res. 122, 339–50.
Gahlmann, R., Troutt, A. B., Wade, R. P., Gunning, P. &Kedes, L. (1987) Alternative splicing generates variants in important functional domains of human slow skeletal troponin T.J. Biol. Chem. 262, 16122–6.
Gambke, B. &Rubinstein, N. (1984) A monoclonal antibody to the embryonic myosin heavy chain of rat skeletal muscle.J. Biol. Chem. 259, 12092–100.
Gusev, N. B., Barskaya, N. V., Verin, A. D., Duzhenkova, I. V., Khuchuha, Z. A. &Zheltova, A. O. (1983) Some properties of cardiac troponin T structure.Biochem. J. 213, 123–9.
Hartner, K. T., Kirschbaum, B. J. &Pette, D. (1989) The multiplicity of troponin T isoforms. Distribution in normal rabbit muscles and effects of chronic stimulation.Eur. J. Biochem. 179, 31–8.
Imai, H., Hirai, S., Hirono, M. &Hirabayashi, T. (1986) Many isoforms of fast muscle troponin T from chicken legs.J. Biochem. (Tokyo) 99, 923–30.
Jin, J. &Lin, J. (1989) Isolation and characterisation of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T.J. Biol. Chem. 264, 14471–7.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227, 680–5.
Medford, R. M., Nguyen, H. T., Destree, A. T., Summers, E. &Nadal-Ginard, B. (1984) A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene.Cell 38, 409–21.
Minty, A. J., Alonso, S., Caravatti, M. &Buckingham, M. (1982) A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA.Cell 30, 185–92.
Moore, G. E., Briggs, M. &Schachat, F. H. (1987) Patterns of troponin T expression in mammalian fast, slow and promiscuous fibres.J. Muscle Res. Cell Motil. 8, 13–22.
Nakamura, M., Imai, H. &Hirabayahi, T. (1989) Coordinate accumulation of troponin subunits in chicken breast muscle.Dev. Biol. 132, 389–97.
Narusawa, M., Fitzsimons, R. B., Izumo, S., Nadal-Ginard, B., Rubinstein, N. &Kelly, A. M. (1987) Slow myosin in developing rat skeletal muscle.J. Cell Biol. 104, 447–59.
Perry, S. V. &Cole, H. (1974) Phosphorylation of troponin and the effects of interactions between the components of the complex.Biochem. J. 141, 733–43.
Sabry, M. A. &Dhoot, G. K. (1989) Identification of and changes in the expression of troponin T isoforms in developing avian and mammalian heart.J. Mol. Cell Cardiol. 21, 85–91.
Saggin, L., Ausosoni, S., Gorza, L., Sartore, S. &Schiaffino, S. (1988) Troponin T switching in the developing rat heart.J. Biol. Chem. 263, 18488–92.
Schachat, F. H., Diamond, M. S. &Brandt, P. W. (1987) The effect of different troponin T-tropomyosin combinations of thin filament activation.J. Mol. Biol. 198, 551–4.
Shimizu, N. &Shimada, Y. (1985) Immunochemical analysis of troponin T isoforms in adult, embryonic, regenerating and denervated chicken fast skeletal muscles.Dev. Biol. 111, 324–34.
Smillie, L. B., Golosinska, K. &Reinach, F. (1988) Sequence of complete cDNAs encoding four variants of chicken skeletal muscle troponin T.J. Biol. Chem. 263, 18816–20.
Stockdale, F. E., Miller, J. B. (1987) The cellular basis of myosin heavy chain expression during development of avian skeletal muscles.Dev. Biol. 123, 1–9.
Sweeney, L. J., Clark, W. A., Umeda, P. K., Zak, R. &Manasek, F. J. (1984) Immunofluorescence analysis of the primordial myosin detectable in embryonic striated muscle.Proc. Natl Acad. Sci. 81, 797–800.
Towbin, H., Staehelin, T. &Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications.Proc. Natl Acad. Sci. 76, 4350–4.
Wilkinson, J. M., Moir, A. J. &Waterfield, M. D. (1984) The expression of multiple forms of troponin T in chicken fast skeletal muscle may result from differential splicing of a single gene.Eur. J. Biochem. 143, 47–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabry, M.A., Dhoot, G.K. Identification of and pattern of transitions of cardiac, adult slow and slow skeletal muscle-like embryonic isoforms of troponin T in developing rat and human skeletal muscles. J Muscle Res Cell Motil 12, 262–270 (1991). https://doi.org/10.1007/BF01745116
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01745116