Summary
Using a variety of techniques, we have demonstrated the presence of at least two fibre types inLimulus median telson levator muscle. By light and electron microscopy, large (21 56 μm2 mean cross-sectional area) fibres have A-bands of 4.1 μm, one-half I bands of 2.15 μm and Z lines ⩽ 0.5 μm in width. Few mitochondria are found in these fibres, which comprise 54% of those present in a given microscope field and which occupy 82% of the total cross-sectional area. Small fibres (484 μm2 mean cross-sectional area) have A bands of 6.3 μm, one-half I bands of 3.1 μm and Z lines between 0.5 and 1.0 μm in width and are rich in mitochondria. Although small fibres comprise nearly one-half (46%) of the fibres in a field, they occupy only 18% of the total cross-sectional area.
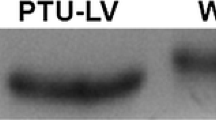
Histochemical staining for alkaline-stable myofibrillar ATPase activity and mitochondrial reduced β-nicotinamide adenine nucleotide (β-NADH) tetrazolium reductase activity confirms the presence of two fibre types. The large fibres react positively for the myofibrillar ATPase activity and negatively for the mitochondrial enzyme activity. The reverse is seen with the small fibres. Some fibres of intermediate size, having intermediate staining characteristics, were also observed. Native gel electrophoresis of both myofibrillar and purified myosin preparations supports the observed differences in myofibrillar ATPase activity in that two myosin isozymes are resolved on pyrophosphate gels. Although the thick filaments isolated from unstimulated small fibres are longer (>6.0 μm) than those isolated from unstimulated large fibres (4.26 μm), all have a similar appearance with respect to the arrangement of myosin heads on their surfaces, and similar diameters. The implications of the observed heterogeneity of fibre types is discussed with reference to previously reported phenomena inLimulus telson muscle, including changes in length of thick filaments on fibre stimulation and the shape of the length-tension curve obtained from fibre bundles.
Similar content being viewed by others
References
Brooke, M. H. &Kaiser, K. K. (1969) Some comments on the histochemical characterization of muscle adenosine triphosphatase.J. Histochem. Cytochem. 17, 431–2.
Crowther, R. A., Padron, R. &Craig, R. (1985) Arrangement of the heads of myosin in relaxed thick filaments from tarantula muscleJ. molec. Biol. 184, 429–39.
Davidheiser, S. &Davies, R. E. (1982) Energy utilization ofLimulus telson muscle at different sarcomere and A-band lengths.Am. J. Physiol. 242, R394-R400.
Davidheiser, S., Levine, R. J. C. &Davies, R. E. (1982) Two different fibre types inLimulus muscle.Fedn Proc. Am. Soc. exp. Biol. 41, 1522 (abstract).
De Villafranca, G. W. (1961) The A- and I-band lengths in stretched or contracted horseshoe crab skeletal muscle.J. Ultrastruct. Res. 5, 109–15.
De Villafranca, G. W. &Marschhaus, C. M. (1963) Contraction of the A-bandJ. Ultrastruct. Res. 9, 157–65.
Dewey, M. M., Levine, R. J. C. &Colflesh, D. E. (1973) Structure ofLimulus striated muscle. The contractile apparatus at various sarcomere lengths.J. Cell. Biol. 58, 574–93.
Dewey, M. M., Walcott, B., Colflesh, D. E., Terry, H. &Levine, R. J. C. (1977) Changes in thick filament length inLimulus striated muscle.J. Cell. Biol. 75, 366–80.
Eisenberg, B. (1983) Quantitative ultrastructure of mammalian skeletal muscle. In:Handbook of Physiology (edited byPeachey, L. D.), pp. 73–112. Bethesda: Am, Physiol. Soc.
Franzini-Armstrong, C. (1970) Natural variability in the length of thin and thick filaments in single fibres from a crab,Portunus depurator.J. Cell Sci. 6, 559–92.
Hoh, J. F., McGrath, P. A. &White, R. I. (1976) Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick.Biochem. J. 157, 87–95.
Kensler, R. W. &Levine, R. J. C. (1982a) An electron microscopic and optical diffraction analysis of the structure ofLimulus thick filaments.J. Cell Biol. 92, 443–51.
Kensler, R. W. &Levine, R. J. C. (1982b) Determination of the hand of the crossbridge helix ofLimulus thick filaments.J. Mus. Res. Cell. Motil. 3, 349–61.
Kensler, R. W., Levine, R. J. C. &Stewart, M. (1985) Electron microscopic and optical diffraction analysis of the structure of scorpion muscle thick filaments.J. Cell. Biol. 101, 395–401.
Kensler, R. W. &Stewart, M. (1986) An ultrastructural study of crossbridge arrangement in the frog thigh muscle thick filament.Biophys. J. 49, 343–51.
Lehman, W., Kendrick-Jones, J. &Szent-Gyorgyi, A. G. (1973) Myosin-linked regulatory systems: comparative studies.Cold Spring Harb. Symp. Quant. Biol. 37, 319–30.
Levine, R. J. C. (1986) Effects of myosin light chain phosphorylation onLimulus thick filaments.J. Cell. Biol. 103, 118a (abstr).
Levine, R. J. C., Davidheiser, S., Kensler, R. W., Kelly, A. M. &Davies, R. E. (1986) Fiber types inLimulus muscle.Biophys. J. 49, 259a (abstr).
Levine, R. J. C., Dewey, M. M. &De Villafranca, G. W. (1972) Immunohistochemical localization of contractile proteins inLimulus striated muscle.J. Cell Biol. 55, 221–36.
Levine, R. J. C. &Kensler, R. W. (1985) Structure of short thick filaments fromLimulus muscle.J. molec. Biol. 182, 347–52.
Levine, R. J. C., Kensler, R. W., Reedy, M. C., Hofmann, W. &King, H. A. (1983) Structure and paramyosin content of tarantula thick filaments.J. Cell Biol. 97, 186–95.
Levine, R. J. C. &Woodhead, J. L. (1987) Structural effects ofLimulus myosin light chain phosphorylation.Biophys. J. 51, 324a (abstr).
Maruyama, K., Sawada, H., Kimura, S., Ohashi, K., Higuchi, H. &Umazume, Y. (1984) Connectin filaments in stretched skinned fibres of frog skeletal muscle,J. Cell. Biol. 99, 1391–7.
Mcneil, P. A. &Hoyle, G. (1967) Evidence for superthin filaments.Am. Zool. 7, 483–98.
Ogonowski, M. M. &Lang, F. (1979) Histochemical evidence for enzyme differences in crustacean fast and slow muscle.J. exp. Zool. 207, 143–54.
Padykula, H. A. &Herman, E. (1955) The specificity of the histochemical method for adenosine triphosphatase.J. Histochem. Cytochem. 3, 170–83.
Rudel, R. &Zite-Ferenczy, F. (1979) Interpretation of light diffraction by cross-striated muscle as a Bragg reflexion of light by the lattice of contractile proteins.J. Physiol., Lond. 290, 317–30.
Sellers, J. R. (1981) Phosphorylation-dependent regulation ofLimulus myosin.J. biol. Chem. 256, 9274–8.
Stewart, M., Kensler, R. W. &Levine, R. J. C. (1981) Structure ofLimulus telson muscle thick filaments.J. molec. Biol. 153, 781–90.
Stewart, M., Kensler, R. W. &Levine, R. J. C. (1985) Three-dimensional reconstruction of thick filaments fromLimulus and scorpion muscle.J. Cell Biol. 101, 402–11.
Trinick, J., Knight, P. &Whiting, A. (1984) Purification and properties of native titin.J. molec. Biol. 180, 331–56.
Walcott, B. &Dewey, M. M. (1980) Length-tension relation inLimulus striated muscle.J. Cell Biol. 87, 204–8.
Wang, K. &Ramirez-Mitchell, R. (1983) A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vetebrate skeletal muscle.J. Cell Biol. 96, 562–70.
Wang, K. &Williamson, C. L. (1980) Identification of an N2 line protein of striated muscle.Proc. Natn. Acad. Sci. U.S.A. 77, 3254–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Levine, R.J.C., Davidheiser, S., Kelly, A.M. et al. Fibre types inLimulus telson muscles: morphology and histochemistry. J Muscle Res Cell Motil 10, 53–66 (1989). https://doi.org/10.1007/BF01739856
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01739856