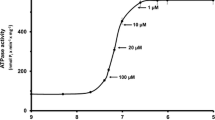
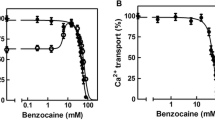
Summary
We have developed a procedure to discriminate actomyosin-type ATPase activity from Ca2+-ATPase activity of sarcoplasmic reticulum (SR) in mechanically skinned fibres, determining simultaneously their Ca2+-induced tension and accompanying ATPase activity. When they were treated with an alkaline CyDTA-containing solution of low ionic strength which was reported to remove troponin C, the fibres showed a considerable amount of Ca2+-dependent ATPase activity, in spite of having little or no Ca2+-induced isometric tension. The residual ATPase activity is ascribed to the Ca2+-ATPase activity of SR, because it is completely abolished by 1% CHAPS treatment for 10 min. This conclusion is also supported by the finding that the Ca2+-dependence of the ATPase activity is very similar to that of Ca2+-ATPase of SR isolated from rabbit skeletal muscle, and that the estimated activity is consistent with the reported values of direct determinations. On the other hand, treatment with a detergent such as CHAPS or Triton X-100 removes SR activities (ATPase and Ca-uptake), leaving Ca2+-induced tension and actomyosin-type ATPase activity unchanged. This procedure indicated that the contribution of Ca2+-ATPase activity of SR may be minimal in total steady-state ATPase activity of mechanically skinned mammalian skeletal muscle fibres. Successive CyDTA and CHAPS treatments eliminated both Ca2+-induced tension and ATPase activity, which were recovered by the addition of troponin C. Using these procedures, we also examined the effect of cyclopiazonic acid (CPA) which was reported to be a specific inhibitor of Ca2+-ATPase of SR. Ca2+-ATPase activity of SR in skinned fibres was inhibited completely by 10 μm CPA and held to one-half by about 0.2 μm. This effect was only partially reversible. CPA at 10μ m or higher concentrations showed Ca2+-sensitizing action on myofibrils, which was readily reversible. CPA at 3μ m inhibited almost completely the Ca2+-ATPase activity of SR, while it had no effect on either actomyosin-type ATPase or isometric tension of myofibrils.
Similar content being viewed by others
References
Duggan, P. F. &Martonosi, A. (1970) Sarcoplasmic reticulum IX. The permeability of sarcoplasmic reticulum membranes.J. Gen. Physiol. 56, 147–67.
Ebashi, S. &Endo, M. (1986) Ca ion and muscle contraction.Prog. Biophys. Mol. Biol. 18, 123–83.
Endo, M. (1977) Calcium release from the sarcoplasmic reticulum.Physiol. Rev. 57, 71–108.
Goeger, D. R., Riley, R. T., Dorner, J. W. &Cole, R. J. (1988) Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles.Biochem. Pharmacol. 37, 978–81.
Goldman, Y. E. (1987) Kinetics of the actomyosin ATPase in muscle fibers.Ann. Rev. Physiol. 49, 637–54.
Guth, K. &Wojciechowski, R. (1986) Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibres.Pflugers Arch. 407, 552–7.
Harafuji, H. &Ogawa, Y. (1980) Re-examination of the apparent binding constant of ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid with calcium around neutral pH.J. Biochem. 87, 1305–12.
Homsher, E. (1987) Muscle enthalpy production and its relationship to actomyosin ATPase.Ann. Rev. Physiol. 49, 673–90.
Inesi, G. (1985) Mechanism of calcium transport.Ann. Rev. Physiol. 47, 573–601.
Kerrick, W. G. L., Secrist, D., Coby, R. &Lucas, S. (1976) Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult.Nature 260, 440–1.
Kurebayashi, N., Kodama, T. &Ogawa, Y. (1980) P1,P5-di(adenosine-5′)pentaphosphate (Ap5A) as an inhibitor of adenylate kinase in studies of fragmented sarcoplasmic reticulum from bullfrog skeletal muscle.J. Biochem. 88, 871–6.
Kurebayashi, H., Ogawa, Y. &Harafuji, H. (1982) Effect of local anesthetics on calcium activated ATPase and its partial reaction with fragmented sarcoplasmic reticulum from bullfrog and rabbit skeletal muscle.J. Biochem. 92, 915–20.
Kurebayashi, N. &Ogawa, Y. (1986) Characterization of increased Ca2+ efflux by quercetin from the sarcoplasmic reticulum in frog skinned skeletal muscle fibres.J. Muscle Res. Cell Motil. 7, 142–50.
Kurebayashi, N. &Ogawa, Y. (1988) Increase by trifluoperazine in calcium sensitivity of myofibrils in a skinned fibre from frog skeletal muscle.J. Physiol. 403, 407–24.
Martonosi, A. N. &Beeler, T. J. (1983) Mechanism of Ca2+ transport by sarcoplasmic reticulum. InHandbook of Physiology, Section 10, Skeletal Muscle (edited by Peachey, L. D., Adrian, R. H. & Geiger, S. R.) pp. 417–85. Bethesda: American Physiological Society.
Morimoto, S. &Ohtsuki, I. (1987) Ca2+- and Sr2+-sensitivity of the ATPase activity of rabbit skeletal myofibrils: effect of complete substitution of troponin C with cardiac troponin C, calmodulin, and parvalbumins.J. Biochem. 101, 291–301.
Ogawa, Y. &Kurebayashi, N. (1982) ATP-ADP exchange reaction by fragmented sarcoplasmic reticulum from bullfrog skeletal muscle.J. Muscle Res. Cell Motil. 3, 39–56.
Ogawa, Y. (1985) Sarcoplasmic reticulum: Ca-pump. InHandbook of Physiological Sciences, Vol. 4,Physiology of Skeletal, Cardiac and Smooth Muscles (edited by Tomita, T. & Sugi, H.) pp. 91–101. Tokyo: Igaku Shoin (in Japanese).
Ogawa, Y. (1988) Comparative aspects of the mechanisms of energy transduction in sarcoplasmic reticulum between rabbit and frog skeletal muscle. InThe Roots of Modern Biochemistry (edited by Kleinkauf, H. Von Döhren, H. & Jaenicke, L.) Pp. 747–58. Berlin, New York: Walter de Gruyter.
Ogawa, Y. &Kurebayashi, N. (1989) Modulations by drugs of the relationship between calcium binding to troponin C and tension. InMuscle Energetics (edited by Paul, R., Elzinga, G. & Yamada, K.) pp. 75–86. New York: Alan R. Liss.
Seidler, N. W., Jona, I., Vegh, M. &Martonosi, A. (1989) Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum.J. Biol. Chem. 264, 17816–23.
Stephenson, D. G. &Williams, D. A. (1981) Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of rat at different temperatures.J. Physiol. 317, 281–302.
Stephenson, D. G., Stewart, A. W. &Wilson, G. J. (1989) Dissociation of force from myofibrillar MgATPase and stiffness at short sarcomere lengths in rat and toad skeletal muscle.J. Physiol. 410, 351–66.
Takagi, A. &Endo, M. (1977) Guinea pig soleus and extensor digitorum longus: a study on single-skinned fibers.Exp. Neurol. 55, 95–101.
Yates, L. D. &Greaser, M. L. (1983) Quantitative determination of myosin and actin in rabbit skeletal muscle.J. Mol. Biol. 168, 123–41.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kurebayashi, N., Ogawa, Y. Discrimination of Ca2+-ATPase activity of the sarcoplasmic reticulum from actomyosin-type ATPase activity of myofibrils in skinned mammalian skeletal muscle fibres: distinct effects of cyclopiazonic acid on the two ATPase activities. J Muscle Res Cell Motil 12, 355–365 (1991). https://doi.org/10.1007/BF01738590
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01738590