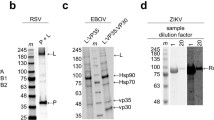
Summary
RNA polymerase of influenza virus with the subunit structure PB1-PB2-PA is involved in both transcription and replication of the genome RNA. The RNA polymerase with transcription activity was reconstituted from three P proteins, which were separately isolated from insect cells infected with recombinant baculoviruses, each carrying cDNA for one P protein. Nuclear extracts of the insect cells infected with each of the recombinant baculoviruses or various combinations of these viruses were examined for transcription and replication activities. The nuclear extract of cells expressing all three P proteins catalyzed model template-directed RNA synthesis in the absence of primers (an indication of RNA replication), supporting the notion that the complete set of three P proteins is required for RNA replication. All the nuclear extracts containing the PB1 subunit, including the extract containing PB1 alone, were able to catalyze model template-directed dinucleotide-primed RNA synthesis (an indication of transcription). These observations not only confirm that the PB1 protein is a catalytic subunit of influenza virus RNA polymerase, but also indicate that PB1 alone is able to catalyze RNA synthesis in the absence of PB2 and PA subunits.
Similar content being viewed by others
References
Akkina RK, Richardson JC, Aguilera MC, Yang C-M (1991) Heterogeneous forms of polymerase proteins exist in influenza A virus- infected cells. Virus Res 19: 17–30
Asano Y, Mizumoto K, Maruyama T, Ishihama A (1995) Photoaffinity labeling of influenza virus RNA polymerase PB1 subunit with 8-azido GTP. J Biochem 117: 677–682
Beaton AR, Krug RM (1984) Synthesis of the templates for influenza virion RNA replication in vitro. Proc Natl Acad Sci USA 81: 4682–4686
Braam J, Ulmanen I, Krug RM (1983) Molecular model of a eukaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell 34: 609–618
Del Rio L, Martinez C, Domingo E, Ortin J (1985) In vitro synthesis of full-length influenza virus complementary RNA. EMBO J 4: 243–247
Digard P, Block VC, Inglis SC (1989) Complex formation between influenza virus polymerase proteins expressed inXenopus oocytes. Virology 171: 162–169
Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 5: 1475–1488
Hay AJ, Lomnicizi B, Bellamy AR, Skehel JJ (1977) Transcription of the influenza virus genome. Virology 83: 337–355
Honda A, Ueda K, Nagata K, Ishihama A (1988) RNA polymerase of influenza virus: Role of NP in RNA chain elongation. J Biochem 104: 1021–1026
Honda A, Mukaigawa J, Yoshikawa A, Kato A, Ueda S, Nagata K, Krystal M, Nayak DP, Ishihama A (1990) Purification and molecular structure of RNA polymerase from influenza virus A/PR8. J Biochem 107: 624–628
Ishihama A, Barbier P (1994) Molecular anatomy of viral RNA-directed RNA polymerases. Arch Virol 134: 235–258
Ishihama A, Mizumoto K, Kawakami K, Kato A, Honda A (1986) Proofreading function associated with the RNA-dependent RNA polymerase from influenza virus. J Biol Chem 261: 10417–10421
Ishihama A, Nagata K (1988) Viral RNA polymerases. CRC Crit Rev Biochem 23: 27–76
Kimura N, Fukushima A, Oda K, Nakada S (1993) An in vitro study of the replication origin in the influenza virus complementary RNA. J Biochem 133: 88–92
Kobayashi M, Tuchiya K, Nagata K, Ishihama A (1992) Reconstitution of influenza virus RNA polymerase from three subunits expressed using recombinant baculovirus system. Virus Res 22: 235–245
Krug RM, Ueda M, Palese P (1975) Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol 16: 790–796
Krug RM, Alonso-Caplen FV, Julkenun I, Katze MG (1989) Expression and replication of the influenza virus genome. In: Krug RM (ed) The influenza viruses. Plenum Press, New York, pp 89–152
Lamb RA (1989) Genes and proteins of the influenza viruses. In: Krug RM (ed) The influenza viruses. Plenum Press, New York, pp 1–87
Luytjes W, Krystal M, Enami M, Parvin JD, Palese P (1989) Amplification, expression and packaging of a foreign gene by influenza virus. Cell 59: 1107–1113
Mahy BWJ, Barrett T, Nichol ST, Penn CR, Wolstenholme AJ (1981) Analysis of the functions of influenza virus genome RNA segments by use of temperature-sensitive mutants of fowl plaque virus, In: Bishop DHL, Compans RW (eds) The replication of negative stranded viruses. Elsevier/North-Holland, New York pp 379–387
Martin J, Albo C, Ortin J, Melero JA, Portela A (1992) In vitro reconstitution of active influenza virus ribonucleoprotein complexes using viral proteins purified from infected cells. J Gen Virol 73: 1855–1859
Matsudaira P (1988) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262: 10035–10038
Mowshowitz SL (1981) RNA synthesis of temperature-sensitive mutants of WSN influenza virus. In: Bishop DHL, Compans RW (eds) The replication of negative stranded viruses. Elsevier/North-Holland, New York, pp 317–323
Murhammer DW, Goochee CF (1988) Scale up of insect cell cultures: protective effects of Pluronic F-68. BioTechnology 6: 1411–1418
Nagata K, Takeuchi K, Ishihama A (1989) In vitro synthesis of influenza viral RNA: biochemical complementation assay of factors required for influenza virus replication. J Biochem 106: 205–208
Nakagawa Y, Kimura N, Toyoda T, Mizumoto K, Ishihama A, Oda K, Nakada (1995) The RNA polymerase PB2 subunit is not required for replication of the influenza virus genome but is involved in capped mRNA synthesis. J Virol 69: 728–733
Parvin JD, Palese P, Honda A, Ishihama A, Krystal M (1989) Promoter analysis of influenza virus RNA polymerase. J Virol 63: 5142–5152
Piccone ME, Fernandes-Sesma A, Palese P (1993) Mutational analysis of the influenza virus vRNA promoter. Virus Res 28: 99–112
Shapiro GI, Krug RM (1988) Influenza virus RNA replication in vitro: synthesis of viral template RNAs and viron RNAs in the absence of an added primer. J Virol 62: 2285–2290
Seong BL, Brownlee GG (1992) Nucleotides 9 to 11 of the influenza A virus RNA promoter are crucial for activity in vitro. J Gen Virol 73: 3115–3124
Seong BL, Brownlee GG (1992) A new method for reconstituting influenza polymerase and RNA in vitro: a study of the promoter elements for cRNA and vRNA synthesis in vitro and viral rescue in vivo. Virology 186: 247–260
Seong BL, Kobayashi M, Nagata K, Brownlee GG, Ishihama A (1992) Comparison of two reconstituted systems for in vitro transcription and replication of influenza virus. J Biochem 111: 496–499
Szewczyk B, Laver WG, Summers DF (1988) Purification, thioredoxin renaturation and reconstituted activity of the three subunits of the influenza A virus RNA polymerase. Proc Natl Acad Sci USA 85: 7907–7911
Takeuchi K, Nagata K, Ishihama A (1987) In vitro synthesis of influenza viral RNA: characterization of an isolated nuclear system that supports transcription of influenza viral RNA. J Biochem 101: 239–256
Toyoda T, Kobayashi M, Ishihama A (1994) Replication in vitro of the influenza virus genome: selective dissociation of RNA replicase from virus-infected cell ribonucleoprotein complexes. Arch Virol 136: 269–286
Ulmanen I, Broni BA, Krug RM (1981) The role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci USA 78: 7355–7359
Ulmanen I, Broni BA, Krug RM (1983) Influenza virus temperature-sensitive cap (m7GpppNm)-dependent endonuclease. J Virol 45: 27–35
Yamanaka K, Ogasawara N, Yoshikawa H, Ishihama A, Nagata K (1991) In vivo analysis of the promoter structure of the influenza virus RNA genome using a transfection system with an engineered RNA. Proc Natl Acad Sci USA 88: 5369–5373
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kobayashi, M., Toyoda, T. & Ishihama, A. Influenza virus PB1 protein is the minimal and essential subunit of RNA polymerase. Archives of Virology 141, 525–539 (1996). https://doi.org/10.1007/BF01718315
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01718315