Abstract
Objective
To determine whether hemofiltration (HF) can eliminate cytokines and complement components and alter systemic hemodynamics in patients with severe sepsis.
Design
Prospective observation study.
Setting
Surgical intensive care unit of a university hospital.
Patients
16 patients with severe sepsis.
Interventions
Continuous zero-balanced HF without dialysis (ultrafiltrate rate 21/h) was performed in addition to pulmonary artery catheterization, arterial cannulation, and standard intensive care treatment.
Measurements and main results
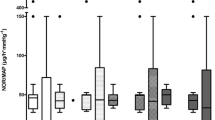
Plasma and ultrafiltrate concentrations of cytokines (the interleukins IL-1β, IL-6, IL-8, and tumor necrosis factor α) and of complement components (C3adesArg, C5adesArg) were measured after starting HF (t0) and 4 h (t4) and 12 h later (t12). Hemodynamic variables including mean arterial pressure (MAP), mean central venous pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, and cardiac output were serially determined. During HF, cytokine plasma concentrations remained constant. However, C3adesArg and C5adesArg plasma concentrations showed a significant decline during 12-h HF (C3adesArg: t0=676.9±99.7 ng/ml vs t12=467.8±71,p<0.01; C5adesArg: 26.6±4.7 ng/ml vs 17.6±6.2,p<0.01). HF resulted in a significant increase over time in systemic vascular resistance (SVR) and MAP (SVR at t0: 669±85 dyne·s/cm5 vs SVR at t12: 864±75,p<0.01; MAP at t0: 69.9±3.5 mmHg vs MAP at t12: 82.2±3.7,p<0.01).
Conclusions
HF effectively eliminated the anaphylatoxins C3adesArg and C5adesArg during sepsis. There was also a significant rise in SVR and MAP during high volume HF. Therefore, HF may represent a new modality for removal of anaphylatoxins and may, thereby, deserve clinical testing in patients with severe sepsis.
Similar content being viewed by others
References
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) The ACCP/SCCM Consensus Conference Committee: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Davenport A, Will E, Davidson AM (1993) Improved cardivascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med 21:328–338
Davenport A, Davision AM, Will E (1993) Membrane biocompatibility: effects on cardiovascular stability in patients on hemofiltration. Kidney Int 43 [Suppl 41]:S230-S234
Deppisch R, Betz M, Hansch GM, Rauterberg EW, Ritz E (1992) Biocompatibility of the polyamide membranes. Contrib Nephrol 96:26–46
Pertosa G, Tarantino E, Gesualdo L, Montinaro V, Schena FP (1993) C5b-9 generation and cytokine production in hemodialyzed patients. Kidney Int 43 [Suppl 41]:S221-S225
Barzilay E, Kessler D, Berlot G, Gullo A, Geber D, Zeev IB (1989) Use of extracorporeal supportive techniques as additional treatment for septic-induced multiple organ failure patients. Crit Care Med 17:634–637
Groeneveld ABJ (1990) Septic shock and multiple organ failure: treatment with hemofiltration?. Intensive Care Med 16:489–490
Bone RC (1991) The pathogenesis of sepsis. Ann Intern Med 115:457–469
Grootendorst AF, van Bommel EFH (1993) The role of hemofiltration in the critically-ill intensive care unit patient: present and future. Blood Purif 11:209–223
Grootendorst AF, van Bommel EFH, van Leengoed LAMG, van Zanten ARH, Huipen HJC, Groeneveld ABJ (1993) Infusion of ultrafiltrate from endotoxemic pigs depresses myocardial performance in normal pigs. J Crit Care 8:161–169
Lee PA, Matson JR, Pryor RW, Hinshaw LB (1993) Continuous arteriovenous hemofiltration therapy forStaphylococcus aureus-induced septicemia in immature swine. Crit Care Med 21:914–924
Storck M, Hartl WH, Zimmerer E, Inthorn D (1991) Comparison of pumpdriven and spontaneous continuous haemofiltration in postoperative acute renal failure. Lancet 337:452–455
Heidemann S, Ofenstein J, Sarnaik A (1993) Efficacy of continuous arteriovenous hemofiltration (CAVH) in endotoxic shock (abstract). Crit Care Med 21:S159
Bellomo R, Tipping P, Boyce N (1993) Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med 21:522–526
Hoffmann JN, Hartl WH, Deppisch R, Faist E, Jochum M, Inthorn D (1995) Hemofiltration in human sepsis: evidence for elimination of immunomodulatory substances. Kidney Int 48:1563–1570
Elebute EA, Stoner HB (1983) The grading of sepsis. Br J Surg 70:29–31
Van Snick J, Cayphas S, Vink A (1989) Purification and NH2-terminal amino acid sequence of a T-cell derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA 83:9679–9683
Espevik T, Nissen-Meyer J (1986) A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods 95:99–105
Eskandari MK, Nguyen DT, Kunkel SL, Remick DG (1990) WEHI subclone 13 assay for TNF: sensitivity, specificity and reliability. Immunol Invest 19:69–79
Smith RA, Baglioni C (1987) The active form of tumor necrosis factor is a trimer. J Biol Chem 262:6951–6954
Onozaki K, Matsushima K, Aggarwal BB, Oppenheim JJ (1985) Human interleukin-1 is a cytocidal factor for several tumor cell lines. J Immunol 135:3962–3968
Tonnesen E, Hansen MB, Höhndorf K, Diamant M, Bendtzen K, Wanscher M, Toft P (1993) Cytokines in plasma and ultrafiltrate during continuous arteriovenous hemofiltration. Anaesth Intensive Care 21:752–758
Millar AB, Armstrong L, van der Linden J, Moat N, Ekroth R, Westwick J, Seallan M, Lincoln C (1994) Cytokine production and hemofiltration in children undergoing cardiopulmonary by-pass. Ann Thorac Surg 56:1499–1502
Journois D, Pouard P, Greeley WJ, Mauriat P, Vouhe P, Safran D (1994) Hemofiltration during cardiopulmonary bypass in pediatric cardiac surgery. Anesthesiology 81:1181–1189
Elliot D, Wiles CE, Reynolds HN (1994) Removal of cytokines in septic patients using continuous veno-venous hemodiafiltration. Crit Care Med 22:718–719
Göhl H, Buck R, Strathmann H (1992) Basic features of the polyamide membranes. Contrib Nephrol 96:1–25
LaMarre J, Wollenberg GK, Gonias SL, Hayes A (1991) Biology of disease: cytokine binding and clearance properties of proteinase-activated alpha2-macroglobulin. Lab Invest 65:3–14
Goldfarb S, Golper TA (1994) Proinflammatory cytokines and hemofiltration membranes. J Am Soc Nephrol 5:228–232
Hakin RM, Wingard RL, Parker RA (1994) Effect of the dialysis membrane in the treatment of patients with acute renal failure. N Engl J Med 331:1338–1342
Barrera P, Janssen EM, Demacker PNM, Wetzels JFM, van der Meer J (1992) Removal of interleukin-1 beta and tumor necrosis factor from human plasma by in vitro dialysis with polyacrlylonitrile membranes. Lymphokine Cytokine Res 11:99–104
Reinhardt B, Steudle V, Krick G (1990) Verfahrenstechnische Aspekte. In: Franz HE (ed) Blutreinigungsverfahren: Technik und Klinik. Thieme, Stuttgart, pp 1–22
MacKenzie SJ, Nimmo GR, Armstrong IG, Grant IS (1991) The hemodynamic effects of intermittent haemofiltration in critically ill patients. Intensive Care Med 17:346–349
Hugli TE (1986) Biochemistry and biology of anaphylatoxins. Complement 13:111
Hack CE, Nuijens JH, Felt-Bersma RJF, Schreuder WO, Eerenberg Belmer AJM, Paardekooper J, Bronsfeld W, Thijs LG (1989) Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med 86:20–26
Haeffner-Cavaillon N, Cavaillon J-M, Laude M, Kazatchkine MD (1987) C3a (C3adesArg) induces production and release of interleukin 1 by cultured human monocytes. J Immunol 139:794–799
Guthrie LA, McPhail LC, Henson PM, Johnston RB (1984) Priming of neutrophils for enhanced release of oxygen metabolites by bacterial polysaccharides. J Exp Med 160:1656–1671
Schirmer WJ, Schirmer JM, Naff GB, Fry DE (1988) Systemic complement activation produces hemodynamic changes characteristic for sepsis. Arch Surg 123:316–321
Groeneveld ABJ, Bronsveld W, Thijs LG (1986) Hemodynamic determinants of mortality in human septic shock. Surgery 99:140–152
Heideman M, Hugli TE (1984) Anaphylatoxin generation in multisystem organ failure. J Trauma 24:1038–1043
Gardinali M, Padalino P, Vesconi S, Calgano A, Ciapellano S, Conciato L, Chirara O, Agostoni A, Nespoli A (1992) Complement activation and polymorphonuclear neutrophil leukocyte elastase in sepsis. Arch Surg 127:1219–1224
Andreasson S, Goethberg S, Berggren H, Bengtsson A, Eriksson E, Risberg B (1993) Hemofiltration modifies complement activation after extracorporeal circulation in infants. Ann Thorac Surg 56:1515–1517
Weisdorf DJ, Hammerschmidt DE, Jacob H (1979) The role of endogenous proteases, circulating granulocytes and the spleen in clearance of activated C5 components: possible relevance to the shock lung syndrome (ARDS). Clin Res 27:649
Volankis JE, Barnum SR, Giddens M, Galla JH (1985) Renal filtration and catabolism of complement protein D. N Engl J Med 312:395–399
Kaiser JP, Götze O, Oppermann M, Deppisch R, Göhl H, Asmus G, Schaefer K (1996) Hemofiltration but not hemodialysis leads to an impressive reduction of factor D in uremia. Blood Purif (in press)
Dofferhoff AS, de Jong HJ, Bom VJ, van der Meer J, Limburg PC, De Vries Hospers HG, Marrink J, Mulder PO, Weits J (1992) Complement activation and the production of inflammatory mediators during the treatment of severe sepsis in humans. Scand J Infect Dis 24:197–204
Hallström S, Koidl B, Müller U, Werdan K, Schlag G (1991) A cardiodepressant factor isolated from blood blocks Ca current in cardiomyocytes. Am J Physiol 260:H869-H876
Hallström S, Bernhardt E, Müller U, Fürst W, Vogl C, Koidl B, Werdan K, Schlag G (1994) A cardiodepressant factor (CDF) isolated from hemofiltrates of patients and/or in cardiogenic shock blocks calcium inward current in cardiomyocytes (abstract). Shock 2:S15
Coraim F, Trubel W, Ebermann R, Werner T (1991) Isolation of low-molecularweight peptides in hemofiltrated patients with cardiogenic shock: a new aspect of myocardial depressant substances. Contrib Nephrol 93:237–240
Bellomo R, McGrath B, Boyce N (1994) Effect of continuous venovenous hemofiltration with dialysis on hormones and catecholamine clearance in critically ill patients with acute renal failure. Crit Care Med 22:833–837
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoffmann, J.N., Hartl, W.H., Deppisch, R. et al. Effect of hemofiltration on hemodynamics and systemic concentrations of anaphylatoxins and cytokines in human sepsis. Intensive Care Med 22, 1360–1367 (1996). https://doi.org/10.1007/BF01709552
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01709552