Summary
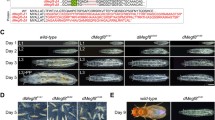
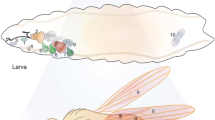
The genecrumbs (crb) ofDrosophila melanogaster provides an essential function for the embryonic development of ectodermally derived epithelia. Complete loss of function alleles of thecrb gene are recessive embryonic lethals and lead to a disorganization of the primordia of these epithelia, followed by cell death in some tissues. Incrb mutant embryos, different organs are affected to a different extent. Some tissues die almost completely (as the epidermis, the atrium and the pharynx) while others partially survive and conserve their basic epithelial structure (as the tracheal system, the oesophagus, the proventriculus, the salivary glands, the hindgut and the Malpighian tubules). Degeneration is first visible at stage 11 and continues successively throughout development. There is evidence that the loss of epithelial cell polarity may be the cause for the degeneration of these tissues, suggesting that thecrb gene product is involved in stabilizing the apico-basal polarity of epithelial cells. As previously shown, thecrb protein is specifically expressed on the apical side of embryonic epithelia in a reticular pattern outlining the borders of the cells. Here we demonstrate that thecrb protein shows the same subcellular localization in epithelial cells of imaginal discs and in follicle cells, indicating a similar function ofcrb during the development of embryonic, imaginal and follicle epithelia. Clonal analysis experiments indicate that the genecrb is not cell-autonomous in its expression, suggesting that the gene product may act as a diffusible factor and may serve as a signal in a cell-cell communication process. This signal is thought to be required for the formation and/or maintenance of the cell and tissue structure of the respective epithelia.
Similar content being viewed by others
References
Akam M (1987) The molecular basis for metameric pattern in theDrosophila embryo. Development 101:1–22
Anderson KV (1987) Dorsal-ventral embryonic pattern genes ofDrosophila. TIG 3:91–97
Becker HJ (1957) Über Röntgenmosaikflecken und Defektmutationen im Auge vonDrosophila und die Entwicklungsphysiologie des Auges. Zeitschrift für indukt. Abstammungs- und Vererbungslehre 88:333–373
Becker HJ (1976) Mitotic Recombination. In: Ashburner M, Wright TRF (eds) The genetics and biology ofDrosophila, vol 1c. Academic Press, New York, pp 1020–1089
Bier E, Vässin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, Jan LY, Jan YN (1989) Searching for pattern and mutations in theDrosophila genome with a P-lacZ vector. Gen Dev 3:1273–1287
Campos-Ortega JA, Hartenstein V (1985) The embryonic development ofDrosophila melanogaster. Springer, Berlin Heidelberg New York Tokyo
Demerec M (Ed.) (1950) Biology ofDrosophila. John Wiley & Sons Inc, New York
Doolittle RF, Feng DF, Johnson MS (1984) Computer-based characterization of epidermal growth factor precursor. Nature 307:558–560
Dura JM, Randsholt NB, Deatrick J, Erk I, Santamaria P, Freeman JD, Freeman SJ, Weddell D, Brock HW (1987) A complex genetic locus,polyhomeotic, is required for segmental specification and epidermal development inD. melanogaster. Cell 51:829–839
Ekblom P (1989) Developmentally regulated conversion of mesenchym to epithelium. The FASEB J 3:2141 2150
Elkins T, Zinn K, McAllister L, Hoffmann FM, Goodman CS (1990) Genetic analysis of aDrosophila neural cell adhesion molecule: Interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell 60:565–575
Fleming TP, Johnson MH (1988) From egg to epithelium. Ann Rev Cell Biol 4:459–485
Foe VE (1989) Mitotic domains reveal early commitment of cells inDrosophila embryos. Development 107:1–22
Fujita SJ, Zipursky SL, Benzer S, Ferrus A, Shotwell SL (1982) Monoclonal antibodies against theDrosophila nervous system. Proc Natl Acad Sci USA 79:7929–7933
Garcia-Bellido A, Merriam JR (1971) Genetic analysis of cell heredity in imaginal discs ofDrosophila melanogaster. Proc Natl Acad Sci USA 68:2222–2226
Ghysen A, O'Kane C (1989) Neural enhancer-like elements are specific cell markers inDrosophila. Development 105:35–52
Gray A, Dull TJ, Ullrich A (1983) The nucleotide sequence of epidermal growth factor cDNA predicts a 128000 molecular weight precursor. Nature 303:722–725
Hartenstein V (1987) The influence of segmental compartmentalisation on the development of the larval peripheral nervous system inDrosophila melanogaster. Roux's Arch Dev Biol 196:101–112
Hartenstein V (1988) Development ofDrosophila larval sensory organs: spatiotemporal pattern of sensory neurones, peripheral axonal pathways and sensilla differentiation. Development 102:869–886
Ingham PW (1988) The molecular genetics of embryonic pattern formation inDrosophila. Nature 335:25–34
Jan LY, Jan YN (1982) Antibodies to horseradish peroxidase as specific neuronal markers inDrosophila and in grasshopper embryos. Proc Natl Acad Sci USA 72:2700–2704
Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H (1984) Mutations affecting the pattern of larval cuticle inDrosophila melanogaster. II. Zygotic loci on the third chromosome. Roux's Arch Dev Biol 193:283–295
King RC (1970) Ovarian development inDrosophila melanogaster. Academic Press, New York London San Francisco
Klein G, Langegger M, Timpl R, Ekblom P (1988) Role of laminin A chain in the development of epithelial cell polarity. Cell 55:331–341
Klingensmith J, Noll E, Perrimon N (1989) The segment polarity phenotype ofDrosophila involves differential tendencies toward transformation and cell death. Dev Biol 134:130–145
Knust E, Dietrich U, Tepaß U, Bremer KA, Weigel D, Vässin H, Campos-Ortega JA (1987a) EGF homologous sequences encoded in the genome ofDrosophila melanogaster, and their relation to neurogenic genes. EMBO J 6:761–766
Knust E, Bremer KA, Vässin H, Ziemer A, Tepaß U, Campos-Ortega JA (1987b) TheEnhancer of split locus and neurogenesis inDrosophila melanogaster. Dev Biol 122:262–273
Lehmann R, Nüsslein-Volhard C (1987)hunchback, a gene required for segmentation of the anterior and posterior region of theDrosophila embryo. Dev Biol 119:402–417
Lewis EB (1978) A gene complex controlling segmentation inDrosophila. Nature 276:565–570
Lindsley DL, Grell EH (1968) Genetic variations ofDrosophila melanogaster. Carnegie Inst Publ no 627, Washington DC
Lindsley DL, Zimm G (1985) The genome ofDrosophila melanogaster. Drosophila Inform Serv 62
Lindsley DL, Zimm G (1990) The genome ofDrosophila melanogaster. Drosophila Inform Serv 68
Livneh E, Glaser L, Segal D, Schlessinger J, Shilo BZ (1985) TheDrosophila EGF receptor homolog: conservation of both hormone binding and kinase domain. Cell 40:599–607
Madhaven MM, Schneiderman HA (1977) Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during the larval development ofDrosophila melanogaster. Roux's Arch Dev Biol 183:269–305
Magrassi L, Lawrence PA (1988) The pattern of cell death infushi tarazu, a segmentation gene ofDrosophila. Development 104:447–451
Martinez-Arias A (1985) The development offused-embryos ofDrosophila melanogaster. J Embryol Exp Morphol 87:99–114
Martinez-Arias A (1989) A cellular basis for pattern formation in the insect epidermis. TIG 5:262–267
Nüsslein-Volhard C, Frohnhöfer HG, Lehmann R (1987) Determination of anteroposterior polarity inDrosophila. Science 238:1675–1681
Perrimon N, Gans M (1983) Clonal analysis of the tissue specificity of recessive female-sterile mutations ofDrosophila melanogaster using a dominant female-sterile mutationFs(1) K1237. Dev Biol 100:365–373
Poodry CA (1980) Epidermis: Morphology and Development. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila, vol 2d. Academic Press, New York, pp 443–498
Price JV, Clifford RJ, Schüpbach T (1989) The maternal ventralizing locustorpedo is allelic tofaint little ball, an embryonic lethal, and encodes theDrosophila EGF receptor homolog. Cell 56:1085–1092
Riggelman B, Wieschaus E, Scheld P (1989) Molecular analysis of thearmadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with aDrosophila segment polarity gene. Gen Dev 3:96–113
Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245:718–725
Scheijter ED, Shilo B-Z (1989) TheDrosophila EGF receptor homolog (DER) gene is allelic tofaint little ball, a locus essential for embryonic development. Cell 56:1093–1104
Schüpbach T (1987) Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo inDrosophila melanogaster. Cell 49:699–707
Scott J, Urdea M, Quiroga M, Sanchez-Pescador R, Fong N, Selby M, Rutter WJ, Bell GI (1983) Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science 221:236–240
Simons K, Fuller SD (1985) Cell surface polarity in epithelia. Ann Rev Cell Biol 1:243–288
Smouse D, Goodman C, Mahowald A, Perrimon N (1988)polyhomeotic: a gene required for the embryonic development of axon pathways in the central nervous system ofDrosophila. Gen Dev 2:830–842
Smouse D, Perrimon N (1990) Genetic dissection of a complex neurological mutant,polyhomeotic, inDrosophila. Dev Biol 139:169–185
Szabad J, Schüpbach T, Wieschaus E (1979) Cell lineage and development in the larval epidermis ofDrosophila melanogaster. Dev Biol 73:256–271
Tearle R, Nüsslein-Volhard C (1987) Tübingen mutants and stocklist. Drosophila Inform Serv 66:209–269
Technau GM (1987) A single cell approach to problems of cell lineage and commitment during embryogenesis ofDrosophila melanogaster. Development 100:1–12
Tepaß U, Theres C, Knust E (1990) TheDrosophila genecrumbs encodes an EGF-like protein expressed on apical membranes ofDrosophila epithelial cells and required for organization of epithelia. Cell 61:787–799
Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF (1988) N-cadherin and integrins: two receptor systems that mediate neural process outgrowth on astrocyte surfaces. Neuron 1:33–43
Van der Meer J (1977) Optical clean and permanent mount preparations for phase contrast microscopy of cuticular structures of insect larvae. Drosophila Inform Serv 52:160
Weigel D, Jürgens G, Küttner F, Seifert E, Jäckle H (1989a) The homeotic genefork head encodes a nuclear protein and is expressed in the terminal regions of theDrosophila embryo. Cell 57:645–658
Weigel D, Bellen HJ, Jürgens G, Jäckle H (1989b) Primordium specific requirement of the homeotic genefork head in the developing gut of theDrosophila embryo. Roux's Arch Dev Biol 198:201–210
Wieschaus E, Riggleman R (1987) Autonomous requirements for the segment polarity genearmadillo duringDrosophila embryogenesis. Cell 49:177–184
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tepaß, U., Knust, E. Phenotypic and developmental analysis of mutations at thecrumbs locus, a gene required for the development of epithelia inDrosophila melanogaster . Roux's Arch Dev Biol 199, 189–206 (1990). https://doi.org/10.1007/BF01682078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01682078