Summary
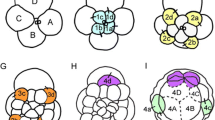
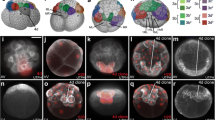
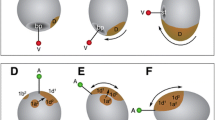
As in many spiralian embryos with unequal cleavage, cleavage inPlatynereis follows an invariant pattern. Preceding each cleavage the cytoplasm is reorganized, allowing the spiral cleavage mode to produce cells with different cytoplasmic composition. The fertilized egg undergoes a dramatic ooplasmic segregation after the completion of the cortical reaction. As a consequence, a plug of clear cytoplasm becomes located at the animal pole. Once the four quadrants of the embryo have been established, the cleavage sequence of the D quadrant differs clearly from that of the other three quadrants. The results presented here suggest that differential distribution of the clear cytoplasm governs this sequence. The first quartet of micromeres, which will form the ectoderm and the cerebral ganglia of the head, is clearly bilaterally symmetrical from the onset of the third cleavage. Dorsoventral polarity and bilateral symmetry in the ectoderm of the trunk is expressed most markedly by the dorsal location of the large 2d cell, whose rapid proliferation is bilaterally symmetrical with respect to the median plane. As a result of this proliferation it comes to fill most of the posttrochal region (ectoderm, three pairs of anlagen for the setal sacs, and the ventral plate which forms the nerve cord). The other micromeres contribute only a minor portion of the ventral ectoderm and are involved in the formation of the stomodaeum. The mesentoblast, 4d, i.e. the stem cell of the primary mesoderm, forms at the sixth cleavage, also in a position on the dorsal mid-line. The daughter cells, which arise from 4d by strictly bilaterally symmetrical cleavage, form the mesodermal germ bands, which lie beneath the ectoderm. The trochoblasts are formed by asynchronously cleaving founder cells, but further cleavages in these cells are synchronous. This suggests that cell-cell interaction is involved in the development of this alleged mosaic embryo.
Similar content being viewed by others
References
Berube G, Powers MM, Kerkay J, Clark GG (1966) The gallocyanin-chrome alum stain: influence of methods of preparation on its activity and separation of active staining compound. Stain Technol 41:73–81
Boilly-Marer Y (1974) Étude expérimentale du comportement nuptial dePlatynereis dumerilii Aud. et M. Edw. (Annelida: Polychaeta): Chémoréception, émission des produits génitaux. Mar Biol 24:167–169
Conklin EG (1912) Cell size and nuclear size. J Exp Zool 12:1–98
Costello DP (1945) Experimental studies of germinal localization inNereis. I. The development of isolated blastomeres. J Exp Zool 100:19–66
Davidson EH (1986) Gene activity in early development, 3rd edn. Academic Press, New York
Dondua AK, Fedorova ZE (1982) Cell cycles at different phases of embryonic and larval development ofNereis virens Sars. Sov J Dev Biol 12:341–347
Dorresteijn AWC, Fischer A (1988) The process of early development. In: Westheide W, Hermans CO (eds) The ultrastructure of Polychaeta. Microfauna Marina vol 4. Gustav Fischer Verlag, Stuttgart, pp 335–352
Dorresteijn AWC, Bornewasser H, Fischer A (1987) A correlative study of experimentally changed first cleavage and Janus development in the trunk ofPlatynereis dumerilii (Annelida, Polychaeta). Roux's Arch Dev Biol 196:51–58
Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T (1983) Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33:389–396
Fischer A (1983) The earliest events in spiralian development (Platynereis, Sabellaria, Pomatoceros). Film C1476. Institut für den Wissenschaftlichen Film, Göttingen, FRG
Götte A (1882) III. Über die Entwicklung der Chätopoden. Abhandlungen zur Entwicklungsgeschichte der Tiere (Leipzig). Heft I:83–104
Hauenschild C, Fischer A (1969)Platynereis dumerilii. Mikroskopische Anatomie, Fortpflanzung, Entwicklung. Großes Zoologisches Praktikum 10b. Gustav Fischer Verlag, Stuttgart
Lillie FR (1912) Studies of fertilization inNereis. III. The morphology of the normal fertilization of Nereis. J Exp Zool 12:413–427
Meijer L, Pondaven P (1988) Cyclic activation of histone H1 kinase during sea urchin egg mitotic divisions. Exp Cell Res 174:116–129
Raven CP (1938) Experimentelle Untersuchungen über die „bipolare Differenzierung“ des Polychaeten- und Molluskeneies. Acta Neerl Morphol Nom Pathol 1:337–357
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Serras F, van den Biggelaar JAM (1987) Is a mosaic embryo also a mosaic of communication compartments? Dev Biol 120:132–138
Spek J (1930) Zustandsänderungen der Plasmakolloide bei Befruchtung und Entwicklung des Nereis-Eies. Protoplasma 9:370–427
Weber R (1958) Über die submikroskopische Organisation und die biochemische Kennzeichnung embryonaler Entwicklungsstadien vonTubifex. Roux' Arch Entwicklungsmech Org 150:542–580
Wilson EB (1892) The cell-lineage ofNereis. A contribution to the cytogeny of the annelid body. J Morphol 6:361–480
Wilson EB (1898) Considerations on cell-lineage and ancestral reminiscence. Ann NY Acad Sci 11:1–27
Wilson EB (1904a) Experimental studies on germinal localization. I. The germ-regions in the egg ofDentalium. J Exp Zool 1:1–72
Wilson EB (1904b) Experimental studies on germinal localization. II. Experiments on the cleavage-mosaic inPatella andDentalium. J Exp Zool 1:197–268
Wilson EB (1925) The cell in development and heredity. 3rd edn with corrections. MacMillan, New York
Zeeck E, Hardege J, Bartels-Hardege H, Wesselmann G (1988) Sex pheromone in a marine polychaete: determination of the chemical structure. J Exp Zool 246:285–292
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dorresteijn, A.W.C. Quantitative analysis of cellular differentiation during early embryogenesis ofPlatynereis dumerilii . Roux's Arch Dev Biol 199, 14–30 (1990). https://doi.org/10.1007/BF01681530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01681530