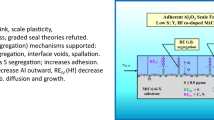
Abstract
Microstructural observations were used as the basis for a discussion of the formation and growth of voids in alumina scales. Reactive-element additions to alloys and alloy desulfurization appear to inhibit the growth of interfacial voids, thus improving scale adhesion. This phenomenon is analyzed in terms of surface energies. In addition, a model is proposed for the formation of large internal voids in α-Al2O3 scales. These voids appear to be too large to form as a result of vacancy coalescence and are more frequently observed in scales not doped with a reactive element. The model is based on a growth mechanism where inward and outward growing ridges at scale grain boundaries eventually seal off and form internal voids.
Similar content being viewed by others
References
T. A. Ramanarayanan, R. Ayer, R. Petkovic-Luton, and D. P. Leta,Oxid. Met. 29, 445 (1988).
M. J. Bennett, H. Romary, and J. B. Price, inHeat Resistant Materials, K. Natesan and D. J. Tillack, eds. (ASM, Materials Park, OH, 1991), pp. 95–103.
E. Tsuzi,Metall. Trans. 11A, 1965 (1980).
J. Smialek and R. Gibala, Diffusion processes in Al2O3 scales: Void growth, grain growth and scale growth, inHigh Temperature Corrosion, R. A. Rapp, ed. (NACE, Houston, TX, 1983), pp. 274–283.
L. W. Hobbs and T. E. Mitchell, Studies of metal oxidation by transmission electron microscopy, inHigh Temperature Corrosion, R. A. Rapp, ed. (NACE,Houston, TX, 1983), pp. 76–83.
D. A. Voss, MS thesis, Case Western Reserve University, Cleveland, OH (1979), from Ref. 5.
P. Kofstad,Oxid. Met. 24, 265 (1985).
C. H. Yang, G. E. Welsch, and T. E. Mitchell,Mater. Sci. Eng. 69, 351 (1985).
P. Choquet and R. Mevrel,Mater. Sci. Eng. A120, 153 (1989).
J. Doychak and M. Ruble,Oxid. Met. 31, 431 (1989).
A. Czyrska-Filemonowicz, R. A. Versaci, D. Clemens, and W. J. Quadakkers, The effect of yttria content on the oxidation resistance of ODS alloys studied by TEM, inMicroscopy of Oxidation 2, S. B. Newcomb and M. J. Bennett, eds. (Institute of Metals, London, U.K., 1993), pp. 288–297.
B. A. Pint, J. R. Martin, and L. W. Hobbs,Oxid. Met. 39, 167 (1993).
B. A. Pint, A. J. Garratt-Reed, and L. W. Hobbs, The effect of a Zr alloy addition on the oxidation behavior ofβ-NiAl: The transition from benefit to breakdown, inMicroscopy of Oxidation 2, S. B. Newcomb and M. J. Bennett, eds. (Institute of Metals, London, U.K., 1993), pp. 463–475.
B. A. Pint and L. W. Hobbs,Oxid. Met. 41, 203 (1994).
B. A. Pint and L. W. Hobbs,J. Electrochem. Soc. 141, 2443 (1994).
B. A. Pint, A. J. Garratt-Reed, and L. W. Hobbs,Mater. High Temp. 13, 3 (1995).
B. A. Pint, Mater.High Temp. (in press).
B. A. Pint, P. F. Tortorelli, and I. G. Wright,Werkst. Korros. 47, 663.
B. A. Pint, The oxidation behavior of oxide dispersedβ-NiAl, (1995) (unpublished research).
B. A. Pint, The high-temperature environmental behavior of oxide-dispersion-strengthened iron-based alloys (1996) (unpublished research).
J. Stringer,Metall. Rev. 11, 113 (1966).
J. E. Antill and K. A. Peakall,J. Iron Steel Inst. 205, 1136 (1967).
V. R. Howes,J. Electrochem. Soc. 116, 1286 (1969).
J. K. Tien and F. S. Pettit,Metall. Trans. 3, 1587 (1972).
M. S. Seltzer, B. A. Wilcox, and J. Stringer,Metall. Trans. 3, 2391 (1972).
A. Kumar, M. Nasrallah, and D. L. Douglass,Oxid. Met. 8, 227 (1974).
C. S. Giggins, B. H. Kear, F. S. Pettit, and J. K. Tien,Metall. Trans. 5, 1685 (1974).
F. A. Golightly, F. H. Stott, and G. C. Wood,Oxid. Met. 10, 163 (1976).
J. L. Smialek,Metall. Trans. 9A, 309 (1978).
J. M. Allam, D. P. Whittle, and J. Stringer,Oxid. Met. 12, 35 (1978).
H. M. Hindam and W. W. Smeltzer,Electrochem. Soc. 127, 1630 (1980).
C. Barrett, A. S. Kahn, and C. E. Lowell,Electrochem. Soc. 128, 25 (1981).
T. Homma, H. M. Hindam, Y. Pyun, and W. W. Smeltzer.Oxid. Met. 17, 223 (1982).
S. Taniguchi and T. Shibata,Oxid. Met. 25, 201 (1986).
E. P. Katz, B. A. Pint, A. J. Garratt-Reed, and G. J. Yurek, The effect of Y2O3 dispersions on the oxidation behavior of ODS alloys (1987) (unpublished research).
V. Provenzano, K. Sadananda, N. P. Louat, and J. R. Reed,Surf. Coatings Technol. 36, 61 (1988).
H. J. Grabke, D. Weimer, and H. Viefhaus,Appl. Surf. Sci. 47, 243 (1991).
P. Y. Hou and J. Stringer,Oxid. Met. 38, 323 (1992).
M. W. Brumm and H. J. Grabke,Corros. Sci. 34, 547 (1993).
B. A. Pint and L. W. Hobbs,Electrochem. Soc. Ext. Abstr. 93-2 676 (1993).
B. A. Pint,Oxid. Met. 45, 1 (1996).
B. A. Pint and C. S. Simpson,Cyclic oxidation of alumina forming alloys at 1300°C (1995) (unpublished research).
B. A. Pint, The Effect of Reactive Elements on the Growth of Al2O3 Scales, PhD thesis, Massachusetts Institute of Technology, Cambridge, MA, 1992.
B. A. Pint, A. J. Garratt-Reed, and L. W. Hobbs,Study of the microstructure of alumina scales (1993) (unpublished research).
H. E. Evans,Int. Mater. Rev. 40, 1 (1995).
H. E. Evans,Mater. High Temp. 12, 219 (1994).
H. E. Evans, J. R. Nicholls, and S. R. J. Saunders,Solid State Phenom. 41, 137 (1995).
M. Schütze,Mater. High Temp. 12, 237 (1994).
P. Hancock and J. R. Nicholls,Mater. Sci. Technol. 4, 398 (1988).
W. D. Kingery, H. K. Bowen, and D. R. Uhlmann,Introduction to Ceramics (John Wiley & Sons, New York, 1976), p. 209.
U. R. Evans,An Introduction to Metallic Corrosion (Edward Arnold, London, 1948), p. 194.
R. Hales and A. C. Hill,Corros. Sci. 12, 843 (1972).
K. Przybylski and G. J. Yurek,Mater. Sci. Forum 43, 1 (1989).
P. Fox, D. G. Lees, and G. W. Lorimer,Oxid. Met. 36, 491 (1991).
H. J. Grabke, G. Kurbatov, and H. J. Schmutzler,Oxid. Met. 43, 97 (1995).
A. W. Funkenbush, J. G. Smeggil, and N. S. Bornstein,Metall. Trans. 16A, 1164 (1985).
J. G. Smeggil, A. W. Funkenbusch, and N. S. Bornstein,Metall. Trans. 17A, 923 (1986).
J. L. Smialek, The effect of sulfur and zirconium co-doping on the oxidation of NiCrAl, inHigh Temperature Materials Chemistry IV, Proc. Vol. 88-5, Z. A. Munir, D. Cubicciotti, and H. Tagawa, eds. (Electrochemical Society, Pennington, NJ, 1988); pp. 241–247.
J. L. Smialek,Metall. Trans. 22A, 739 (1991).
G. H. Meier, F. S. Pettit, and J. L. Smialek,Werkst. Korros. 46, 232 (1995).
B. A. Pint, I. G. Wright, W. Y. Lee, Y. Zhang, K. Prübner, and K. B. Alexander, Substrate and Bond Coat Compositions: Factors Affecting Alumina Scale Adhesion, submitted toMaterials, Science, and Engineering (1997).
E. Schumann, J. C. Yang, M. J. Graham, and M. Rühle,Werkst. Korros. 46, 218 (1995).
E. Schumann, J. C. Yang, M. Rühle, and M. J. Graham,Oxid. Met. 46, 37 (1996).
J. G. Smeggil, E. L. Paradis, A. J. Shuskus, and N. S. Bornstein,J. Vac. Sci. Technol. A 3, 2569 (1985).
D. R. Sigler,Oxid. Met. 32, 337 (1989).
D. Ting and W. Longmei,J. Less-Common Met. 110, 179 (1985).
K. A. Gschneider and N. Kippenham,Thermochemistry of the Rare Earch Carbides, Nitrides, and Sufides for Steelmaking (Rare Earth Information Center, Institute for Atomic Research, Iowa State University, Ames, IA, 1972).
J. D. Harrison and C. Wagner,Acta Metall. 7, 722 (1959).
G. J. Yurek, Interface Stability During the Oxidation of Binary Alloys, ORNL Report #5116, Oak Ridge National Laboratory, Oak Ridge, TN (1976).
M. Bobeth, W. Pompe, and M. Rockstroh, Modelling of the structural development of oxide scales on Ni3A1 single crystals during the initial stage of oxidation, inMicroscopy of Oxidation 2, S. B. Newcomb and M. J. Bennett, eds. (Institute of Metals, London, U.K., 1993), pp. 412–422.
R. A. Rapp,Metall. Trans. 15A, 765 (1984).
J. Shen and R. A. Rapp,Electrochem. Soc. Ext. Abstr. 93-2, 672 (1993).
J. E. Harris,Met. Sci. 12, 321 (1978).
W. J. Quadakkers, H. Holzbrecher, K. G. Briefs, and H. Beske,Oxid. Met. 32, 67 (1989).
K. P. R. Reddy, J. L. Smialck, and A. R. Cooper,Oxid. Met. 17, 429 (1982).
A. H. Heuer, N. J. Tighe, and R. M. Cannon,J. Am. Ceram. Soc. 63, 53 (1980).
I. G. Wright, W. Y. Lee, and B. A. Pint, High temperature corrosion issues in advanced gas turbines systems intended for power generation, inCorrosion `96 (NACE, Houston, TX, 1996), Paper #96143.
B. A. Pint, L. W. Hobbs,Oxid. Met. 47, 1.
J. Doychak, J. L. Smialek, and C. A. Barrett, Oxidation of Ni-Rich Ni-Al intermetallics, inOxidation of High-Temperature Intermetallics, T. Grobstein and J. Doychak, eds. (TMS, Warrendale, PA, 1988), pp. 41–55.
B. A. Pint, J. R. Martin, and L. W. Hobbs,Solid State Ionics 78, 99 (1995).
B. A. Pint, A. J. Garratt-Reed, and L. W. Hobbs, Analytical electron microscopy study of the duplex scale formed on an ODS Ni3A1 alloy, inMicroscopy of Oxidation 2, S. B. Newcomb and M. J. Bennett, eds. (Institute of Metals, London, U.K., 1993), pp. 423–434.
S. B. Shendye and D. A. Downham,Oxid. Met. 43, 435 (1995).
M. J. Bennett, M. R. Houlton, and G. Dearnaley,Corros. Sci. 20, 69 (1980).
K. L. Luthra and C. L. Briant,Metall. Trans. 19A, 2091 (1988).
A. J. Garratt-Reed, Analysis of boundaries, inProceedings of the 45th Annual Meeting of the Electron Microscopy Society of America, G. Bailey, ed. (San Francisco Press, San Francisco, 1987), pp. 300–303.
A. J. Garratt-Reed, Measurement of segregation at interfaces by AEM, inProceedings of the 50th Annual Meeting of the Electron Microscopy Society of America, G. W. Bailey, J. Bentley, and J. A. Small, eds. (San Francisco Press, San Francisco, CA, 1992), pp. 1206–1207.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pint, B.A. On the formation of interfacial and internal voids inα-Al2O3 scales. Oxid Met 48, 303–328 (1997). https://doi.org/10.1007/BF01670505
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01670505