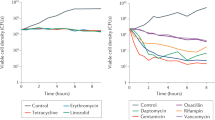
Summary
The responses of bacteria exposed to amoxycillin and ampicillin were studied at continuously decreasing levels with half-life values similar to those which could occur in vivo. ForEscherichia coli, the kill-rates were higher with amoxycillin than with ampicillin. The bactericidal response was exponential. With an antibiotic half-life of one hour, the amoxycillin first order inactivation rate was 3.544 h−1 and the viable cell half-life was 0.196 h; the respective values for ampicillin were 2.341 h−1 and 0.296 h. With an antibiotic half-life of five hours, the inactivation rate was 0.704 h−1 corresponding to a viable cell half-life of 0.985 h for amoxycillin compared to 0.358 h−1 and 1.937 h respectively for ampicillin. Comparison of viable counts and photometric monitoring showed that the former is the preferable method for recording the bacterial response to these β-lactam antibiotics. During the phase of exponential kill, a plateau occurred in the optical density values. This was due in part to an increased biomass per cell. During the recovery phase, the number of viable cells started to increase several hours sooner than did the rise in optical density. ForStaphylococcus aureus, the rates of kill were similar with both agents. Amoxycillin had a long bacteriostatic phase which was not seen with ampicillin. This led to a longer lasting antibacterial effect and reduction to a lower total count with amoxycillin. With staphylococci, the viable counts and the photometric responses were parallel.
Zusammenfassung
Der antibakterielle Effekt von Amoxicillin und Ampicillin in kontinuierlich abfallenden Konzentrationen, deren Halbwertzeiten In-vivo-Bedingungen entsprechen, wurde untersucht. Die Abtötungsraten vonEscherichia coli waren größer mit Amoxicillin als mit Ampicillin und verliefen exponentiell. Bei einer Halbwertzeit von einer Stunde betrug die Amoxicillin-Inaktivierungsrate erster Ordnung 3,544 h−1 und die Halbwertzeit der Lebendzellzahl war 0,196 h; die entsprechenden Werte betrugen für Ampicillin 2,341 h−1 bzw. 0,296 h. Bei einer Antibiotika-Halbwertzeit von fünf Stunden belief sich die Inaktivierungsrate auf 0,704 h−1 mit einer entsprechenden Halbwertzeit der Lebendzellzahlen von 0,985 h für Amoxicillin im Vergleich zu 0,358 h−1 bzw. 1,937 h für Ampicillin. Ein Vergleich der Lebendzellzahl-Bestimmung mit der photometrischen Messung ergibt, daß die erstere Methode zur Erfassung des Effekts von β-Laktam-Antibiotika auf Bakterien besser geeignet ist. Während der exponentiellen Abtötungsphase tritt ein Plateau in den Dichtemessungen auf. Dieser Effekt ist teilweise auf eine Zunahme der zellulären Biomassen zurückzuführen. Während der Erholungsphase nehmen die Lebendzellzahlen einige Stunden früher zu als der Wiederanstieg der optischen Dichte. GegenüberStaphylococcus aureus waren die Abtötungsraten beider Substanzen vergleichbar. Amoxicillin wies eine lange bakteriostatische Phase auf, welche mit Ampicillin nicht beobachtet werden konnte. Dies führte zu einem länger anhaltenden antibakteriellen Effekt und einer größeren Reduktion der Gesamtzellzahl mit Amoxicillin. Mit Staphylokokken verliefen die Lebendzellzahl-Bestimmungen und die photometrischen Messungen parallel.
Similar content being viewed by others
Literatur
Croydon, E. A. P., Sutherland, R. Microbiology and human pharmacology of amoxycillin (BRL 2333): In:Hejzlar, M., Semonsky, M., Masak, S. (eds.): Advances in Antimicrobial and Antineoplastic Chemotherapy, Vol. 1. Urban & Schwarzenberg. München, 1972, p. 975–979.
Duval, J., Soussy, C. J. Activité antibactérienne et pharmacocinétique de l'amoxicilline. Comparaison avec l'ampicilline. Méd. Malad. Infect. 12 (1973) 525–531.
Finland, M., McGowan, J. E., Garner, C., Wilcox, C. Amoxicillin: In vitro susceptibility of „blood culture strains“ of gramnegative bacilli and comparisons with penicillin G, ampicillin, and carbenicillin. J. Infect. Dis. 129 Suppl. (1974) 132–138.
Handsfield, H. H., Clack, H., Wallace, J. F., Holmes, K. K., Turck, M. Amoxicillin, a new penicillin antibiotic. Antimicrob. Agents Chemother. 3 (1973) 262–265.
Neu, H. C. Antimicrobial activity and human pharmacology of amoxicillin. J. Infect. Dis. 129 Suppl. (1974) 123–131.
Sutherland, R., Croydon, E. A. P., Rolinson, G. N. Amoxycillin: A new semi-synthetic penicillin. Br. Med. J. II (1972) 13–16.
Lode, H., Janisch, P., Küpper, G., Weuta, H. Comparative clinical pharmacology of three ampicillins and amoxicillin administered orally. J. Infect. Dis. 129 Suppl. (1974) 156–168.
Sjövall, J., Magni, J., Bergan, T. Pharmacokinetics of bacampicillin compared with those of ampicillin, pivampicillin, and amoxycillin. Antimicrob. Agents Chemother. 13 (1978) 90–96.
May, J. R., Ingold, A. Amoxicillin in the treatment of infections of the lower respiratory tract. J. Infect. Dis. 129 Suppl. (1974) 189–193.
Stewart, S. M., Anderson, I. M. E., Jones, G. R., Calder, M. A. Amoxycillin levels in sputum, serum, and saliva. Thorax. 29 (1974) 110–114.
Stewart, S. M., Fisher, M., Young, J. E., Lutz, W. Ampicillin levels in sputum, serum, and saliva. Thorax 25 (1970) 304–311.
Tan, J. S., Bannister, T., Phair, J. P. Levels of amoxicillin and ampicillin in human serum and interstitial fluid. J. Infect. Dis. 129 Suppl. (1974) 146–148.
Acred, P., Hunter, P. A., Mizen, L., Rolinson, G. N. α-Amino-p-hydroxybenzylpenicillin (BRL 2333), a new broad-spectrum semisynthetic penicillin: In vivo evaluation. Antimicrob. Agents Chemother. 1970 (1971) 416–422.
Acred, P., Brown, D. M., Clark, B. F., Mizen, L. The distribution of antibacterial agents between plasma and lymph in the dog. Br. J. Pharmacol. 39 (1970) 439–446.
Basker, M. J., Gwynn, M. N., White, A. R. Comparative activities of ampicillin, epicillin and amoxycillin in vitro and in vivo. Chemotherapy 25 (1979) 170–180.
Comber, K. R., Boon, R. J., Sutherland, R. Comparative effects of amoxycillin and ampicillin on the morphology ofEscherichia coli in vivo and correlation with activity. Antimicrob. Agents Chemother. 12 (1977) 736–744.
Hunter, P. A., Rolinson, G. N., Witting, D. A. Comparative activity of amoxycillin and ampicillin in an experimental bacterial infection in mice. Antimicrob. Agents Chemother. 4 (1973) 285–293.
Brogden, R. N., Speight, T. M., Avery, G. S. Amoxycillin: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 9 (1975) 88–140.
Ohkoshi, M. Evaluation of amoxycillin in urology. In: Amoxycillin (BRL 2333). Excerpta Medica, Amsterdam 1974, p. 39–47.
Ritzerfeld, W. Neue antibakterielle Substanzen im Tierversuch. Zbl. Bakteriol. 240 (1974) 330–332.
Dalhoff, A. 50 Jahre Penicilline. Eine Übersicht über Struktur-Wirkungsbeziehungen derβ-Lactamantibiotika und ihre mikrobiologische sowie klinische Relevanz. Infection 7 (1979) 294–302.
Rolinson, G. N., MacDonald, A. C., Wilson, D. A. Bactericidal action ofβ-lactam antibiotics onEscherichia coli with particular reference to ampicillin and amoxycillin. J. Antimicrob. Chemother. 3 (1977) 541–553.
Fukui, M., Nakazawa, S. Study on mechanism of antibacterial action of amoxycillin. J Chemother. (Japan) 22 (1974) 1111–1114.
Bergan, T., Carlsen, J. B., Fuglesang, J. An in vitro model monitoring bacterial responses to antibiotic agents at simulated in vivo conditions. Infection 8 Suppl. I (1980) 96–102.
Ericsson, H. M. & Sherris, J. C.: Antibiotic sensitivity testing. Report of an international collaborative study. Acta Path. Microbiol. Scand. Sect. B. Suppl. 217 (1971).
Reyn, A., Bentzon, M. W., Ericsson, H. Comparative investigations on the sensitivity ofN. gonorrhoeae to penicillin. Acta Path. Microbiol. Scand. 57 (1963) 235–255.
Greenwood, D. Response profiles: A method of evaluating the activity ofβ-lactam antibiotics against enterobacteria. Chemotherapy 23 (1977) 11–18.
Helm, E. B. Antibakterielle Aktivität von Antibiotika in Körperflüssigkeiten. Johann A. Wülfing, Neuss, West-Germany, 1977.
Greenwood, D., O'Grady, F. Comparison of the responses ofEscherichia Coli andProteus mirabilis to sevenβ-lactam antibiotics. J. Infect. Dis. 128 (1973) 211–221.
McDonald, P. J., Craig, W. A., Kunin, C. M. Brief antibiotic exposure and effect on bacterial growth: In:Williams, J. D., Geddes, A. M. (eds.): Chemotherapy, Vol. 2. Laboratory Aspects of Infections. Plenum Press, New York, 1976, p. 95–102.
McDonald, P. J., Craig, W. A., Kunin, C. M. Persistent effect of antibiotics ofStaphylococcus aureus after exposure for limited periods of time. J. Infect. Dis. 135 (1977) 217–223.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergan, T., Carlsen, I.B. Bacterial kill rates of amoxycillin and ampicillin at exponentially diminishing concentrations simulating in vivo conditions. Infection 8 (Suppl 1), S103–S108 (1980). https://doi.org/10.1007/BF01644943
Issue Date:
DOI: https://doi.org/10.1007/BF01644943