Summary
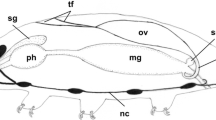
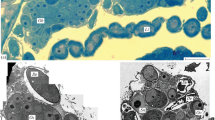
Histological and ultrastructural investigations of diplopod ovary structure have revealed that oogonia and early meiotic oocytes develop only in the laterodorsal parts of the ovarian wall, where they form groups called germ nests. Euplasmic growth forces diplotene oocytes out of the ovarian wall and into the lumen of the ovary, which leads to the formation of ovarian sacs. Ovarian sacs constitute separate structural-functional units built of a centrally situated oocyte and the epithelial cover. Being turned with their basal parts to the surface of the oocyte and showing no signs of any synthetic nor secretory activity, the epithelial cells of the ovarian sac wall cannot be referred to as typical follicular cells. That is why oogenesis of diplopods must be regarded as solitary.
Similar content being viewed by others
References
Beams HW, Kessel RG (1969) Synthesis and deposition of oocyte envelopes (vitelline membrane, chorion) and the uptake of yolk in the dragonfly (Odonata, Aeschnidae). J Cell Sci 4:241–264
Biliński Sz (1979) Ultrastructural studies on oogenesis in Symphyla. Previtellogenic and vitellogenic stages. Cell Tissue Res 202:145–153
Biliński Sz (1983) Oogenesis inCampodea sp. (Insecta, Diplura). Chorion formation and the ultrastructure of follicle cells. Cell Tissue Res 228:165–170
Biliński Sz, Klag J (1982) Gap junctions between oocyte and follicle cells inAcerentomon sp. (Insecta, Protura). Int J Invert Reprod 5:331–335
Biliński Sz, Petrysak B (1978) The ultrastructure and function of follicle cells inFoucartia squamulata (Herbst.) (Curculionidae). Cell Tissue Res 189:347–353
Biliński Sz, Hage WJ, Bluemink JG (1985) Gap junctions between the follicular cells and the oocyte during oogenesis in an insect,Tribolium destructor (Coleoptera). Roux's Arch Dev Biol 194:296–300
Brennan MD, Weiner AJ, Góralski TJ, Mahowald AP (1982) The follicle cells are a major site of vitellogenine synthesis inDrosophila melanogaster. Dev Biol 89:225–236
Crane DF, Cowden RR (1968) A cytochemical study of oocyte growth in four millipedes. Z Zeilforsch 90:414–431
Cummings MR, Brown NM, King RC (1971) The cytology of the vitellogenic stages of oogenesis inDrosophila melanogaster. III. Formation of the vitelline membrane. Z Zellforsch 118:482–492
Fabre M (1855) Recherches sur l'anatomie des organes reproducteurs et sur developpement de Myriapode. Ann Sci Nat Zool 4:257–316
Herbaut C (1974) Etude cytochimique et origine des enveloppes ovocytaires chezLithobius forficatus L. (Myriapode, Chilopode). Symp Zool Soc. London 32:237–247
Huebner E, Injeyan H (1981) Follicular modulation during oocyte development in an insect: formation and modification of septate and gap junctions. Dev Biol 83:101–113
Jura C (1975) Ovaries structure inAcerentomon dispar Stach. (Protura). Acta Biol Cracov Ser Zool XVIII: 55–65
Nadarajalingam K, Subramoniam T (1982) Oogenesis inSpirostreptus asthenes (Pocock) (Myriapoda, Diplopoda). Zool Anz 212:229–239
Newport G (1841) On the organs of reproduction and the development of the Myriapoda. Phil Trans R Soc 1841:99–130
Reynolds EW (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Sareen ML, Adiyodi KG (1983) Oogenesis, ooposition and oosorption 19. Arthropoda-Myriapoda. In: Adiyodi KG, Adiyodi RG (eds) Reproductive Biology of Invertebrates. John Wiley, London New York
Seifert G (1960) Die Entwicklung vonPolyxenus lagurus L. (Diplopoda, Pselaphognatha). Zool Jb Anat Ont 78:257–312
Telfer WH, Huebner E, Smith DS (1982) The biology of vitellogenic follicles inHyalophora andRhodnius. In: King RC, Akai H (eds) Insect ultrastructure (1). Plenum Press, New York
Tiegs OW (1940) The embryology and affinities of the Symphyla, based on a study ofHanseniella agilis. Q J Microsc Sci 82:1–225
Tiegs OW (1947) The development and affinities of the Pauropoda, based on a study ofPauropus silvaticus p. I. Q J Microscop Sci 88/2:165–269
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubrakiewicz, J. Ultrastructural investigation of the ovary structure ofOphyiulus pilosus (Myriapoda, Diplopoda). Zoomorphology 110, 133–138 (1991). https://doi.org/10.1007/BF01632869
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01632869