Summary
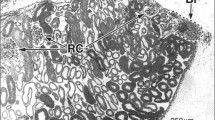
The ultrastructure of the scaphopod kidney and secretory product composition is described, for the first time, inDentalium rectius. The kidney epithelium consists of two primarily secretory cell types. The first exhibits extensive vacuolation, and scattered granules are formed within the vacuolar space by a process of surface accretion; the incorporation of glycogen particles in this process is associated with very fine, electronopaque threads which radiate from the granule. The second cell type possesses granules enclosed individually within secretory vesicles, and intermediate stages in their growth are characterized by needle-like crystals on the granule surface. The secretory vesicles in some cases coalesce to form a large central vacuole filled with granules. This cell type possesses an apical membrane with sparse microvilli, which may indicate a secondary reabsorptive capacity. Granules in both cell types show a concentric ring ultrastructure, and are composed primarily of calcium phosphate with a small amount of zinc; there is also an organic component of protein, mucopolysaccharide and a large amount of glycogen. Ultrastructural and histochemical observations indicate a lysosomal origin for the granules, although granules of the second cell type develop intracellularly to a greater extent than those of the first. All granules are extruded into the kidney lumen by a process of merocrine secretion prior to release into the mantle cavity via an externally ciliated, muscular excretory pore.
Similar content being viewed by others
References
Andrews EB (1976) The ultrastructure of the heart and kidney of the pilid gastropod molluscMarisa cornuarietis, with special reference to filtration throughout the Architaenioglossa. J Zool 179:85–106
Andrews EB (1979) Fine structure in relation to function in the excretory system of two species ofViviparus. J Molluscan Stud 45:186–206
Andrews EB (1981) Osmoregulation and excretion in prosobranch gastropods. Part 2: structure in relation to function. J Molluscan Stud 47:248–289
Andrews EB (1985) Structure and function in the excretory system of archaeogastropods and their significance in the evolution of gastropods. Philos Trans R Soc London Ser B 310:383–406
Andrews EB (1988) Excretory systems of Molluscs. In: Trueman ER, Clarke MR (eds) The Mollusca, vol 11. Academic Press, New York, pp 381–448
Boissevain M (1904) Beiträge zur Anatomie und histologie vonDentalium. Jena Z Naturwiss 38:553–572, plates xvii–xix
Brown BE (1982) The form and function of metal-containing “granules” in invertebrate tissues. Biol Rev Philos Soc Cambridge 57:621–667
Bryan GW (1973) The occurrence and seasonal variation of trace metals in the scallopsPecten maximus (L.) andChlamys opercularis (L.). J Mar Biol Assoc UK 53:145–166
Carmichael NG, Squibb KS, Fowler BA (1979) Metals in the molluscan kidney: a comparison of two closely related bivalve species (Argopecten), using X-ray microanalysis and atomic absorption spectroscopy. J Fish Res Board Can 36:1149–1155
Chapman DM (1977) Eriochrome cyanin as a substitute for haematoxylin and eosin. Can J Med Technol 39:65–66
Chayen J, Bitensky L, Butcher RG (1973) Practical Histochemistry. Wiley, London
Delhaye W (1976) Histophysiologie comparée des organes rénaux chez les archaeogastéropodes (Mollusca — Prosobranchia). Cah Biol Mar 17:305–322
Distaso A (1905) Sull' Anatomia degli scafopodi. Zool Anz 29:271–278
Doyle LJ, Blake NJ, Woo CC, Yevich P (1978) Recent biogenic phosphorite: concretions in mollusk kidneys. Science 199:1431–1433
Fol H (1885) Sur l'anatomie microscopique du Dentale. CR Acad Sci Ser D 1885:1352–1355
Fol H (1889) Sur l'anatomie microscopique du Dentale. Arch Zool Exp Gen 7:91–148, plates 5–8
George SG (1982) Subcellular accumulation and detoxication of metals in aquatic animals. In: Physiological mechanisms of marine pollutant toxicity. Academic Press, New York, pp 3–52
George SG, Pirie BJS (1980) Metabolism of zinc in the mussel,Mytilus edulis (L.): a combined ultrastructural and biochemical study. J Mar Biol Assoc UK 60:575–590
George SG, Pirie BJS, Cheyne AR, Coombs TL, Grant PT (1978) Detoxification of metals by marine bivalves: an ultrastructural study of the compartmentation of copper and zinc in the oysterOstrea edulis. Mar Biol 45:147–156
George SG, Pirie BJS, Coombs TL (1980) Isolation and elemental analysis of metal rich granules from the kidney of the scallop,Pecten maximus (L.). J Exp Mar Biol Ecol 42:143–156
George SG, Coombs TL, Pirie BJS (1982) Characterization of metal-containing granules from the kidney of the common mussel,Mytilus edulis. Biochim Biophys Acta 716:61–71
Hopkin SP, Nott JA (1979) Some observations on concentrically structured, intracellular granules in the hepatopancreas of the shore crabCarcinus maenas (L.). J Mar Biol Assoc UK 59:867–877
Humason GL (1979) Animal Tissue Techniques, 4th edn. Freeman, San Francisco
Kowalevsky A (1889) Ein Beitrag zur Kenntnis der Exkretionsorgane. Biol Zentralbl 9(3):65–76
Lacaze-Duthiers (de) H (1857) Histoire de l'organisation et du développement du Dentale. Ann Sci Nat Zool Biol Anim 4° 7:5–51, plates 2–4; 171–255, plates 5–9
Leeson CR, Leeson TS (1970) Staining methods for sections of epon-embedded tissues for light microscopy. Can J Zool 48:189–191
Lillie RD (1965) Histopathologic Technic and Practical Histochemistry, 3rd edn. McGraw-Hill, New York
Little C (1965) The formation of urine by the prosobranch gastropod molluscViviparus viviparus Linn. J Exp Biol 43:39–54
Little C (1972) The evolution of kidney function in the Neritacea (Gastropoda, Prosobranchia). J Exp Biol 56:249–261
Little C (1979) Reabsorption of glucose in the renal system ofViviparus. J Molluscan Stud 45:207–208
Lowe DM, Moore MN (1979) The cytochemical distributions of zinc (Zn II) and iron (Fe III) in the common mussel,Mytilus edulis, and their relationship with lysosomes. J Mar Biol Assoc UK 59:851–858
Martin AW (1983) Excretion. In: Saleuddin ASM, Wilbur KM (eds) The Mollusca, vol 5, part 2. Academic Press, New York, pp 353–405
Mason AZ, Nott JA (1980) The association of the blood vessels and the excretory epithelium in the kidney ofLittorina littorea (L.) (Mollusca, Gastropoda). Mar Biol Lett 1:355–365
Mason AZ, Simkiss K (1983) Interactions between metals and their distribution in tissues ofLittorina littorea (L.) collected from clean and polluted sites. J Mar Biol Assoc UK 63:661–672
Mason AZ, Simkiss K, Ryan KP (1984) The ultrastructural localization of metals in specimens ofLittorina littorea collected from clean and polluted sites. J Mar Biol Assoc UK 64:699–720
Moore MN (1977) Lysosomal responses to environmental chemicals in some maine (sic) invertebrates. In: Giam CS (ed) Pollutant effects on marine organisms. Lexington Books, Lexington, pp 143–154
Morse MP (1987) Comparative functional morphology of the bivalve excretory system. Am Zool 27(3):737–746
Odhner NH (1931) Die Scaphopoden. In: Bock S (ed) Further Zoological Results of the Swedish Antarctic Expedition 1901–1903, vol 2, no 5, pp 1–8, plates 1, 2
Overnell J (1981) Protein and oxalate in mineral granules from the kidney ofPecten maximus (L.). J Exp Mar Biol Ecol 52:173–183
Pearse AGE (1968) Histochemistry, Theoretical and Applied, vol 1, 3rd edn. Churchill, London
Pearse AGE (1972) Histochemistry, Theoretical and Applied, vol 2, 3rd edn. Churchill Livingston, London
Pelseneer P (1899) Recherches morphologiques et phylogenetiques sur les mollusques archaïques. Mem CI Sci Acad R Belg Collect 4° 57(3):1–113, plates 1–24
Pirie BJS, George SG (1979) Ultrastructure of the heart and excretory system ofMytilus edulis (L.). J Mar Biol Assoc UK 59:819–829
Plate L (1888) Bemerkungen zur Organisation der Dentalien. Zool Anz 11:509–515
Plate L (1892) Ueber den Bau und die Verwandtschaftsbeziehungen der Solenoconchen. Zool Jahrb Abt Anat Ontog Tiere 5:301–386, plates 23–26
Potts WTW (1967) Excretion in the Molluscs. Biol Rev Philos Soc Cambridge 42:1–41
Potts WTW (1975) Excretion in the Gastropods. Fortschritte der Zoologie 23(2/3):76–88
Reid RGB, Fankboner PV, Brand DG (1984) Studies of the physiology of the giant clamTridacna gigas Linné-II. Kidney function. Comp Biochem Physiol 78A(1):103–108
Reynolds PD (1990) Functional morphology of the perianal sinus and pericardium ofDentalium rectius (Mollusca: Scaphopoda) with a reinterpretation of the scaphopod heart. Am Malacol Bull 7(2):137–146
Simkiss K (1976) Intracellular and extracellular routes in biomineralization. In: Duncan CJ (ed) Calcium in biological systems: Symposia of the Society for Experimental Biology no. 30. Cambridge University Press, London, pp 423–444
Simkiss K, Mason AZ (1983) Metal ions: metabolic and toxic effects. In: Hochachka PW (ed) The Mollusca, vol 2. Academic Press, New York, pp 101–164
Sullivan PA, Robinson WE, Morse MP (1988) Isolation and characterization of granules from the kidney of the bivalveMercenaria mercenaria. Mar Biol 99:359–368
Thomson JD, Pirie BJS, George SG (1985) Cellular metal distribution in the Pacific oyster,Crassostrea gigas (Thun.) determined by quantitative X-ray microprobe analysis. J Exp Mar Biol Ecol 85:37–45
Tiffany WJ (1982) Excretory concretions in the sunray venus clam,Macrocallista nimbosa (Bivalvia: Veneridae). Veliger 25(1):77–79
Turchini J (1923) L'excretion urinaire chez les Mollusques. Chap 5, Scaphopodes. In: Dion, G (ed) Contribution à l'étude de l'histologie comparée de la cellule rénale. Librairie Octave Dion, Paris, pp 114–115
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reynolds, P.D. Fine structure of the kidney and characterization of secretory products inDentalium rectius (Mollusca, Scaphopoda). Zoomorphology 110, 53–62 (1990). https://doi.org/10.1007/BF01632812
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01632812