Abstract
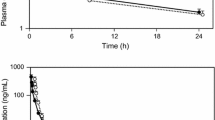
Clinical studies have been performed to investigate the pharmacokinetics and pharmacodynamics of alendronate, an inhibitor of bone resorption for the treatment of osteoporosis. Alendronate is one of the most potent bisphosphonates currently undergoing clinical investigation (>100-fold more potent than etidronate in vivo). The pharmacokinetics of alendronate are similar to those of other bisphosphonates. After a 2-h intravenous infusion, plasma concentrations of alendronate decline rapidly to ∼5% of initial values within 6 h. About 50% of a systemic dose is excreted unchanged in the urine in the 72 h following administration. By analogy to its behavior in animals the remainder is assumed to be taken up by the skeleton. After sequestration into bone, the elimination of alendronate is very prolonged. The terminal half-life was estimated to be greater than 10 years. Despite prolonged skeletal residence, the biological effects of alendronate begin to diminish post-treatment, since the duration of effect reflects factors besides dose and cumulative drug exposure. When taken after an overnight fast, 2 h before breakfast, the oral bioavailability of alendronate averages ∼0.75% of dose with substantial variability (coefficient of variation 55%–75%) both between and within subjects. Reducing the wait before food from 2 h to 1 h, or even 30 min, produces a mean reduction in absorption of 40%. Since the clinical efficacy of alendronate is indistinguishable whether it is given 30 min, 1h, or 3 h before a meal, the observed variability in bioavailability within this range is of little consequence. Dosing up to at least 2 h after a meal dramatically reduces absorption (80%–90%).
Similar content being viewed by others
References
Fleisch H. Bisphosphonates: pharmacology and use in the treatment of tumour-induced hypercalcemia and metastatic bone disease. Drugs 1991;42:919–44.
Schenk R, Eggli P, Fleisch H, et al. Quantitative morphometric evaluation of the inhibitory activity of new aminobisphosphonates on bone resorption in the rat. Calcif Tissue Int 1986;38:342–9.
Sato M, Grasser W. Effects of bisphosphonates on isolated rat osteoclast as examined by reflected light microscopy. J Bone Miner Res 1990;5:31–40.
Sato M, Grasser W, Endo N, et al. Bisphosphonate action: alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 1991;88:1–11.
Adami S, Salvagno G, Guarrear G, et al. Treatment of Paget's disease of bone with intravenous 4-amino-1-hydroxybutylidene-1,1-bisphosphonate. Calcif Tissue Int 1986; 39:226–9.
Bickerstaff DR, O'Doherty DP, McClosky EV, et al. Effects of amino-butylidene diphosphonate in hypercalcemia due to malignancy. Bone 1991;12:17–20.
Zysset E, Ammann P, Jenzer A, et al. Comparison of a rapid (2-hr) versus a slow (24-hr) infusion of alendronate in the treatment of hypercalcemia of malignancy. Bone Miner 1992;18:237–9.
Attardo-Parinello G, Merlini G, Pavesi F, et al. Effects of a new aminodiphosphonate (aminohydroxybutylidene diphosphonate) in patients with osteolytic lesions from metastases and myelomatosis. Arch Intern Med 1987;147:1629–33.
Passeri M, Baroni MC, Pedrazzoni M, et al. Intermittent treatment with intravenous 4-amino-1-hydroxybutylidene-1,1-bisphosphonate (AHBuBP) in the therapy of postmenopausal osteoporosis. Bone Miner 1991;15:237–48.
O'Doherty DP, Gertz BJ, Tindale W, et al. Effects of five daily 1 hr infusions of alendronate in Paget's disease of bone. J Bone Miner Res 1992;7:81–7.
Kline WF, Matuszewski BK, Bayne WF. Determination of 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid in urine by automated pre-column derivatization with 2,3-naphthalene dicarboxyaldehyde and high-performance liquid chromatography with fluorescence detection. J Chrom Bio Med Appl 1990;534:139–49.
Lin JH, Duggan DE, Chen I-W, Ellsworth RL. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos 1991;19:926–32.
Lin JH, Chen I-W, Duggan DE. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab Dispos 1992;20:473–8.
Lin JH, Chen I-W, deLuna Fa, Hichens M. Renal handling of alendronate in rats: an uncharacterized renal transport system. Drug Metab Dispos 1992;20:608–13.
Troehler U, Bonjour J-P, Fleisch H. Renal secretion of diphosphonates in rats. Kidney Int 1975;8:6–13.
Thompson DD, Seedor JG, Weinreb M, et al. Aminohydroxybutane bisphosphonate inhibits bone loss due to immobilization in rats. J Bone Miner Res 1990;5:279–86.
Apseloff G, Girten B, Walker M, et al. Aminohydroxybutane bisphosphonate prevents bone loss in a rat model of simulated weightlessness. Curr Therap Res 1991;50:794–803.
Balena R, Yamamoto M, Gentile M, et al. The aminobisphosphonate alendronate inhibits bone loss induced by thyroid hormone in the rat: comparison between effects on tibiae and vertebrae. Bone 1993 (in press).
Seedor JG, Quartuccio HA, Thompson DD. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 1991;6:339–46.
Toolan BC, Shea M, Myers ER, et al. The effect of long-term alendronate treatment on vertebral strength in ovariectomized baboons and rats. J Bone Miner Res 1991;6(Suppl 1):S48.
Thompson DD, Seedor JG, Quartuccio H, et al. The bisphosphonate, alendronate, prevents bone loss in ovariectomized baboons. J Bone Miner Res 1992;7:951–60.
Fern ED, Vasikaran SD, Khan S, et al. Sustained suppression of bone resorption after intravenous alendronate in postmenopausal osteoporosis. In: 4th International Symposium of Osteoporosis, Hong Kong, March 1993.
Gertz BJ, Kline WF, Matuszewski BK, et al. Oral bioavailability and dose proportionality of alendronate in postmenopausal women. J Bone Miner Res 1991;6(Suppl 1):S279.
Gertz BJ, Kline WF, Holland SD, Matuszewski BK, et al. The effect of gastric pH on oral absorption of alendronate sodium. In: 4th International Symposium on Osteoporosis, Hong Kong, March 1993.
Burssens A, Gertz BJ, Francis RM, Tucci JR, et al. A double-blind, placebo-controlled, rising multiple dose trial of oral alendronate in Paget's disease. J Bone Miner Res 1990;5(Suppl 2):S239.
Harris ST, Gertz BJ, Eyre DR, et al. The effect of short-term treatment with alendronate upon vertebral density and biochemical markers of bone remodeling in early postmenopausal women. J Clin Endocrinol Metab 1993;76:1399–1406.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gertz, B.J., Holland, S.D., Kline, W.F. et al. Clinical pharmacology of alendronate sodium. Osteoporosis Int 3 (Suppl 3), 13–16 (1993). https://doi.org/10.1007/BF01623002
Issue Date:
DOI: https://doi.org/10.1007/BF01623002