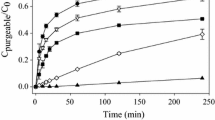
Conclusions
The sulfate component of sea salts is inocuous. However, if reduced to sulfide in anaerobic sediments, it severely reduces the availability of Hg++ for methylation by methylcobalamin. The bicarbonate component of sea salts noticably slows the methylation of Hg++ under both aerobic and anaerobic conditions. In the presence of bicarbonate, other sea salts anions have no significant influence on the methylation of Hg++. In the dark, monomethylmercuric chloride (CH3HgCl) is chemically stable in the presence of all tested seawater anions, including sulfide.
Similar content being viewed by others
References
BERTILSSON, L., and H. Y. NEUJAHR: Biochemistry10:2805 (1971).
BLUM, J., and R. BARTHA: Bull. Environm. Contam. Toxicol.25:404 (1980).
BRINCKMAN, F. E. and W. P. IVERSON: pp. 319–342. In: T. Church (ed.).Marine Chemistry in the Coastal Environment. ACS Symp. 18, Washington, D.C. 1975.
CHU, V. C. W., and D. GRUENWEDEL: Bioinorg. Chem.7:169 (1977).
DADDARIO, J. J.: Bull. N.J. Acad. Sci.6:7 (1961).
D'ITRI, P. A. and F. M. D'ITRI: Environ. Manage.2(1):3 (1978).
DOLPHIN, D.: pp. 34–52. In: D. B. McCormick and L. D. Wright (eds.).Methods in Enzymology. Vol. XVIII Part C, Academic Press, New York. 1971.
FAGERSTROM, T., and A. JERNELOV: Water Res.5:121 (1971).
FURAKAWA, K., and K. TONOMURA: Agric. Biol. Chem.35:604 (1971).
FURAKAWA, K., and K. TONOMURA: Agric. Biol. Chem.36:217 (1972a).
FURAKAWA, K., and K. TONOMURA: Agric. Biol. Chem.36:2441 (1972b).
HAHNE, H. C. H., and W. KROONTJE: J. Environ. Qual.2(4):444 (1973).
IMURA, N., E. SUKEGAWA, S. K. PAN, K. NAGOA, J. Y. KIM, T. KWANANA, T. UKITA: Science172:1248 (1971).
JENSEN, S., and A. JERNELOV: Nature223:753 (1969).
RIDLEY, W. P., L. J. DIZIKES, and J. M. WOOD: Science197:329 (1977).
ROBINSON, G. C., F. NOME, and J. FENDLER: J. Amer. Chem. Soc.99:4969 (1977).
STOTZKY, G., and H. BABICH: Crit. Rev. Micro.8(2):99 (1980).
TAIT, R. V., and R. S. DE SANTO:Elements of Marine Ecology. Springer-Verlag, New York 1972.
TALMI, Y., and R. E. MESMER: Water Res.9:547 (1975).
THAYER, J.: Inorg. Chem.20:3573 (1981).
WOOD, J. M.: Science183:1049 (1974).
YAMADA, M., and K. TONOMURA: J. Ferment. Technol.50:159 (1972a).
YAMADA, M., and K. TONOMURA: J. Ferment. Technol.50:893 (1972b).
YAMADA, M., and K. TONOMURA: J. Ferment. Technol.50:901 (1972c).
Author information
Authors and Affiliations
Additional information
New Jersey Agricultural Experiment Station Publication No. D-01504-1-83 (NJSG-83-112), supported by State Funds and by Sea Grant NOAA NA 81 AA-D-00065, R/E-5.
Rights and permissions
About this article
Cite this article
Compeau, G., Bartha, R. Effects of sea salt anions on the formation and stability of methylmercury. Bull. Environ. Contam. Toxicol. 31, 486–493 (1983). https://doi.org/10.1007/BF01622282
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01622282