Summary
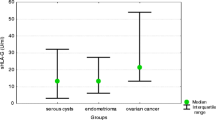
To assess the role of interleukin-1β (IL-1β) and tumour necrosis factorα (TNFα) in the physiological host defence mechanisms against malignancies, the production of these cytokines in sera, ascitic and cyst fluids and in the tumour tissues of patients with benign or malignant ovarian tumours was studied. IL-1β was found neither in the sera nor in the ascitic fluids of these patients. It was also virtually absent from the cyst fluid samples. However, a mean value of 790 pg IL-1β/g tumour was found. Like IL-1β, TNFα was virtually absent in the serum samples. It was, however, detectable in the ascitic and cyst fluids and tumour tissues. The TNFα concentrations were highest in the tumour tissues, with a mean level of 328 pg/g tumour. When comparing the level of IL-1β and TNFα in patients with benign tumours to that seen in patients with malignant tumours, no differences in production were observed, regardless of the origin of the test samples. Our results indicate the production of IL-1β and TNFα in patients with ovarian tumours. More importantly, the finding that the production of these cytokines in patients with benign tumours is similar to that in patients with malignant tumours supports the conclusion that the production of these cytokines is more a nonspecific indicator of an inflammatory process than a specific response to a malignant process.
Similar content being viewed by others
Abbreviations
- IL:
-
interleukin
- TNF:
-
tumour necrosis factor
References
Abbruzzese JL, Levin B, Ajani JA, Faintuch JS, Saks S, Patt YZ, Edwards C, Ende K, Gutterman JU (1989) Phase I trial of recombinant human gamma-interferon and recombinant human tumor necrosis factor in patients with advanced gastrointestinal cancer. Cancer Res 49:4057–4061
Aderka D, Fisher S, Levo Y, Holtman H, Hahn T, Wallach D (1985) Cachectin/tumour-necrosis-factor production by cancer patients. Lancet II:1190
Aderka D, Levo Y, Ramot B, Michalevicz R, Meytes D, Shaklai M, Hahn T, Holtman H, Revel M, Wallach D (1987) Reduced production of tumor necrosis factor by mononuclear cells in hairy cell leukemia patients and improvement following interferon therapy. Cancer 60:2208–2212
Balkwill F, Burke F, Talbot D, Tavernier J, Osborne R, Naylor S, Durbin H, Fiers W (1987) Evidence for tumour necrosis factor/cachectin production in cancer. Lancet II:1229–1232
Beissert S, Bergholz M, Waase I, Lepsien G, Schauer A, Pfizenmaier K, Kronke M (1989) Regulation of tumor necrosis factor gene expression in colorectal adenocarcinoma: in vivo analysis by in situ hybridization. Proc Natl Acad Sci USA 86:5064–5068
Belardelli F, Proietti E, Ciolli V, Sestili P, Carpinelli G, Di Vito M, Ferretti A, Woodrow D, Boraschi D, Podo F (1989) Interleukin-1beta induces tumor necrosis and early morphologic and metabolic changes in transplantable mouse tumors. Similarities with the anti-tumor effects of tumor necrosis factor alpha and beta. Int J Cancer 44:116–123
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA 72:3666–3670
Crump III WL, Owen-Schaub LB, Grimm EA (1989) Synergy of human recombinant interleukin 1 with interleukin 2 in the generation of lymphokine-activated killer cells. Cancer Res 49:149–153
Dejana E, Bertocchi F, Bortolami MC, Regonesi A, Tonta A (1988) Interleukin 1 promotes tumor cell adhesion to cultured human endothelial cells. J Clin Invest 82:1466–1470
Dinarello CA (1987) The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol Lett 16:227–232
Giovini FS di, Duff GW (1990) Interleukin 1: the first interleukin. Immunol Today 11:13–20
Haranaka K, Satomi N, Sakurai A (1984) Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int J Cancer 34:263–267
Holtman H, Walach D (1987) Down regulation of the receptors for tumor necrosis factor by interleukin 1 and 4beta-phorbol-12-myristate-13-acetate. J Immunol 139:1161–1167
Ishii Y, Uchiyama Y, Hasegawa S, Kinoshita T, Mitsui K, Kojima H, Fujita T (1990) Detection of tumor necrosis factor/cachectin in pleural effusion of patients with lung cancer. Clin Exp Immunol 80:350–353
Larsson E-V, Iscove NN, Coutinho A (1980) Two distinct factors are required for induction of T-cell growth. Nature 283:664–666
Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N (1987) Macrophage-induced angiogenesis is mediated by tumor necrosis factor-α. Nature 329:630–632
Lewis GD, Aggarwal BB, Eessalu TE, Sugarman BJ, Shepard HM (1987) Modulation of the growth of transformed cells by human tumor necrosis factor-alpha and interferon-gamma. Cancer Res 47:5382–5385
Lindemann A, Ludwig W-D, Oster W, Mertelsmann R, Herrmann F (1989) High-level secretion of tumor necrosis factor-α contributes to hematopoietic failure in hairy cell leukemia. Blood 73:880–884
Malik STA, Griffin DB, Fiers W, Balkwill FR (1990) Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur J Cancer 26:1031–1034
Merigan TC (1988) Human interferon as a therapeutic agent. N Engl J Med 318:1458–1460
Moritz T, Niederle N, Baumann J, May D, Kurschel E, Osieka R, Kempeni J, Schlick E, Schmidt CG (1989) Phase I study of recombinant tumor necrosis factor-α in advanced malignant disease. Cancer Immunol Immunother 29:144–150
Nathan CF (1987) Secretory products of macrophages
Naylor MS, Malik STA, Stamp GWH, Jobling T, Balkwill FR (1990) In situ detection of tumour necrosis factor in human ovarian cancer specimens. Eur J Cancer 26:1027–1030
Onozaki K, Matsushima K, Aggarwal BB, Oppenheim JJ (1985a) Human interleukin 1 is cytocidal for several tumor cell lines. J Immunol 135:3962–3968
Onozaki K, Matsushima K, Kleinerman ES, Saito T, Oppenheim JJ (1985b) Role of interleukin 1 in promoting human monocytemediated tumor cytotoxicity. J Immunol 135:314–320
Oppenheim JJ, Kovacs EJ, Matsushima K, Durum SK (1986) There is more than one interleukin 1. Immunol Today 7:45–56
Ostensen ME, Thiele DL, Lipsky PE (1987) Tumor necrosis factor-α enhances cytolytic activity of human natural killer cells. J Immunol 138:4185–4191
Owen-Schaub LB, Gutterman JU, Grimm EA (1988) Synergy of tumor necrosis factor and interleukin 2 in the activation of human cytotoxic lymphocytes: effect of tumor necrosis factorα and interleukin 2 in the generation of human lymphokine-activated killer cell cytotoxicity. Cancer Res 48:788–792
Owen-Schaub LB, Crump WL, Morin GI, Grimm EA (1989) Regulation of lymphocyte tumor necrosis factor receptors by IL-2. J Immunol 143:2236–2241
Philip R, Epstein LB (1988) Tumor necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself,γ-interferon and interleukin 1. Nature 323:86–89
Reiter Z, Rubinstein M (1990) Interleukin-1α and tumor necrosis factor-α protect cells against natural killer cell mediated cytotoxicity and natural killer cytotoxic factor. Cell Immunol 125:326–336
Rosenberg SA (1988) Immunotherapy of cancer using interleukin 2: current status and future prospects. Immunol Today 9:58–62
Saarinen U, Koskelo E-K, Teppo A-M, Siimes MA (1990) Tumor necrosis factor in children with malignancies. Cancer Res 50:592–595
Scuderi P, Sterling KE, Lam KS, Finley PR, Ryan KJ, Ray CG, Petersen E, Slymen DJ, Salmon SE (1986) Raised serum levels of tumor necrosis factor in parasitic infections. Lancet 11:1364–1365
Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Shepard HM (1985) Recombinant human tumor necrosis factor-α: effects on proliferation of normal and transformed cells in vitro. Science 230:943–945
Waage A, Espevik T, Lamvik J (1986) Detection of tumour necrosis factor-like cytotoxicity in serum from patients with septicaemia but not from untreated cancer patients. Scand J Immunol 24:739–743
Weiss C, Stehle B, Ho AD, Hunstein W (1990) Serum levels of tumor necrosis factor-α in hairy cell leukemia (letter). Blood 75:321–322
Winkelhake JL, Stampfl S, Zimmerman RJ (1987) Synergistic effects of combination therapy with human recombinant interleukin-2 and tumor necrosis factor in murine tumor models. Cancer Res 47:3948–3953
Yang SC, Owen-Schaub L, Mendiguren-Rodriques A, Grimm EA, Hong WK, Roth JA (1990) Combination immunotherapy for non-small cell lung cancer. J Thorac Cardiovasc Surg 99:8–13
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Punnonen, J., Heinonen, P.K., Kuoppala, T. et al. Production of interleukin-1β and tumour necrosis factor-α in patients with benign or malignant ovarian tumours. J Cancer Res Clin Oncol 117, 587–592 (1991). https://doi.org/10.1007/BF01613293
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01613293