Abstract
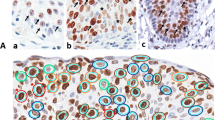
DNA topoisomerase type II (DT-II) is a major component of interphase nuclear matrix fractions, present in S-phase of the cell cycle. A series of 80 carcinomatous breast surgical samples was evaluated by immunohistochemistry, using a polyclonal antibody in a comparison with Ki-67 antiserum. A correlation with clinico-pathological data was also performed. Infiltrating ductal and lobular carcinomas constantly express DT-II with varying intensity of nuclear staining; a similar immunohistochemical pattern is observed with Ki-67. A frequent co-expression of DT-II and Ki-67 is encountered with double immunostaining; accordingly to these data, a linear relationship is evident when linear regression is employed. In addition, significant relationships between DT-II values and tumour size, histological grade and node involvement are shown, while an inverse correlation is appreciable between DT-II and oestrogen receptors and progesterone receptors. DT-II may be considered to be an additional operational marker for the proliferating fraction of cells in breast carcinomas.
Similar content being viewed by others
References
Barnard NJ, Hall PA, Lemoine NR, Kadar N (1987) Proliferative index in breast carcinoma determined in situ by Ki-67 immunostaining and its relationship to clinical and pathological variables. J Pathol 152:287–295
Berrios M, Osheroff N, Fisher PA (1985) In situ localization of DNA topoisomerase II, a major polypeptide component of theDrosophila nuclear matrix fraction. Proc Natl Acad Sci USA 82:4142–4146
Bloom HJG, Richardson WW (1957) Histological grading and prognosis in breast cancer. Br J Cancer 11:359–377
Bodley AL, Wu HY, Liu LF (1987) Regulation of DNA topoisomerases during cellular differentiation. Monogr Natl Cancer Inst 4:31–35
Bouzubar N, Walker KJ, Griffiths K (1989) Ki-67 immunostaining in primary breast cancer: pathological clinical associations. Br J Cancer 59:943–947
Chow KC, Ross WE (1987) Topoisomerase-specific drug sensitivity in relation to cell cycle progression. Mol Cell Biol 7:3119–3123
Duguet M, Lavenot C, Harper H, Mirambeau G, De Recondo AM (1983) DNA topoisomerases from rat liver: physiological variations. Nucleic Acids Res 11:1059–1075
Epstein RJ, Smith PJ, Watson JV, Waters C, Bleehen NM (1989) Oestrogen potentiates topoisomerase II mediated cytotoxicity in an activated subpopulation of human breast cancer cells: implications for cytotoxic drug resistance in solid tumours. Int J Cancer 44:501–505
Fonatsch C, Duchrow M, Rieder H (1991) Assignment of the human Ki-67 gene (MK 167) to 10q25-qter. Genomics 11:476–477
Gerdes J, Lelle RJ, Pickartz H (1986) Growth fraction in breast cancers determined in situ with monoclonal antibody Ki-67. J Clin Pathol 39:977–980
Gerdes J, Pickartz H, Brotherton J, Hammerstein J, Weitzel H, Stein H (1987) Growth fractions and estrogen receptors in human breast cancers as determined in situ with monoclonal antibodies. Am J Pathol 129:486–492
Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD (1991) Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 138:867–873
Hall PA, Levison DA (1990) Assessment of cell proliferation in histological material. J Clin Pathol 43:184–192
Hall PA, Woods AL (1990) Immunohistological markers of cell proliferation: achievements, problems and prospects. Cell Tissue Kinet 23:531–549
Harland RM, Weintraub H, McKnight SL (1983) Transcription of DNA injected intoXenopus oocytes is influenced by template topology. Nature 302:38–43
Heck MMS, Earnshaw WC (1986) Topoisomerase II: a specific marker for cell proliferation. J Cell Biol 103:2569–2581
Hsiang Y, Wu HY, Liu LF (1988) Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res 48:3230–3235
Kaguni JM, Kornberg A (1984) Replication initiated at the origin (ori C) of theE. coli chromosome reconstituted with purified enzymes. Cell 38:183–190
Lelle' RJ, Heidenreich W, Stauch G, Gerdes J (1987) The correlation of growth fraction with histologic grading and lymph node status in human mammary carcinoma. Cancer 59:83–88
Liu LF (1983) DNA topoisomerases: enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem 15:1–24
Liu LF (1989) DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem 58:351–375
Luchnik AN, Bakayev VV, Zbarsky IB, Georgief GP (1982) Elastic torsional strain in DNA within a fraction of SV40 minichromosomes: relation to transcriptionally active chromatin. EM-BO J 1:1353–1358
Marchetti E, Querzoli P, Marzola A, Bagni A, Ferretti S, Fabris G, Nenci I (1990) Assessment of proliferative rate of breast cancer by Ki-67 monoclonal antibody. Mod Pathol 3:31–35
Mason DY, Sammons R (1978) Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol 31:454–460
Nelson EM, Tewey KM, Liu LF (1984) Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4′-(9-acridinylamino)-methanesulfon-m-amsidine. Proc Natl Acad Sci USA 81:1361–1365
Potmesil M (1988) DNA topoisomerase II as intracellular target in anthracycline treatment of cancer. In: Lown JW (ed) Anthracyclines and anthracenedione-based anticancer agents. Elsevier New York.
Potmesil M, Hsiang YH, Liu LF, Bank B, Grossberg H, Kirschenbaum S, Forlenzar T, Penziner A, Kanganis D, Knowles D, Traganos F, Silber R (1988) Resistance of human leukemic and normal lymphocytes to drug-induced DNA cleavage and low levels of DNA topoisomerase II. Cancer Res 48:3537–3543
Raymond WA, Leong AS-Y (1989) The relationship between growth fractions and oestrogen receptors in human breast carcinoma, as determined by immunohistochemical staining. J Pathol 158:203–211
Smith PJ, Makinson TA (1989) Cellular consequences of overproduction of DNA topoisomerase II in an ataxia-telangiectasia cell line. Cancer Res 49:1118–1124
Sullivan DM, Glisson BS, Hodges PK, Smallwood-Kentro S, Ross WE (1986) Proliferation dependence of topoisomerase II mediated drug action. Biochemistry 25:2248–2256
Tan KB, Dorman TE, Falls KM, Chung TDY, Mirabelli CK, Crooke ST, Mao J (1992) Topoisomerase II alfa and topoisomerase II beta genes: characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Res 53:231–234
Tandon AK, Hilsenbeck SG, Clark GM, Allred DC, Latham MD, Ross WE, McGuire WL (1991) Significance of topoisomerase II in clinical breast cancer. Proc Am Assoc Cancer Res 32:350
Tandou G, Mirambeau G, Lavenot G, Garabedian A der, Vermeersch J, Duguet M (1984) DNA topoisomerase activities in concanavalin A-stimulated lymphocytes. FEBS Lett 176:431–435
Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF (1983) Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 226:466–468
Tricoli JV, Sahai BM, McCormick PJ, Sarlinski SJ, Bertram JS, Kowalski D (1985) DNA topoisomerase I and II activities during cell proliferation and the cell cycle in cultured mouse embryo fibroblast (C3H10T1/2) cells. Exp Cell Res 158:1–14
Verheijen R, Kuijpers HJH, Van Driel R (1989) Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J Cell Sci 92:531–540
Wang JC (1985) DNA topoisomerases. Annu Rev Biochem 54:665–697
Wrba F, Reiner A, Markis-Ritzinger E, Holzner JH, Reiner G, Spona J (1988) Prognostic significance of immunohistochemical parameters in breast carcinomas. Pathol Res Pract 183:277–283
Yang L, Rowe TC, Liu LF (1985) Identification of DNA topoisomerase II as an intracellular target of antitumor epipodophyllotoxins in Simian Virus 40-infected monkey cells. Cancer Res 45:5872–5876
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tuccari, G., Rizzo, A., Giuffre', G. et al. Immunocytochemical detection of DNA topoisomerase type II in primary breast carcinomas: Correlation with clinico-pathological features. Vichows Archiv A Pathol Anat 423, 51–55 (1993). https://doi.org/10.1007/BF01606432
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01606432