Summary
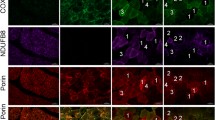
Previous studies have revealed an increase of cytochrome c oxidase-deficient fibres/cells in the skeletal and heart muscle of humans during ageing. The enzyme defect is due to a lack of both mitochondrial and nuclear coded enzyme subunits. In the present investigation in situ hybridization of mitochondrial DNA (mtDNA) has been performed on extraocular muscles of humans over 70 years of age to show whether mutated mtDNA with the so called common deletion of 4,977 basepairs at position 8,482–13,460 of mtDNA accumulates in the cytochrome c oxidase-deficient fibres. The cytochrome c oxidase-deficient fibres revealed different hybridization patterns: a normal hybridization signal with three different mtDNA probes, a reduced or lacking signal with all three probes indicating depletion of mtDNA and a selective hybridization defect with the probe recognizing the “common deletion” region of mtDNA as evidence of mtDNA deletion. The results suggest that during ageing defects of cytochrome c oxidase are associated with different molecular alterations of mtDNA. Deletion and depletion of mtDNA are not the only nor probably the leading mechanisms responsible for the loss of respiratory chain capacity during ageing. The normal hybridization signal in most of the cytochrome c oxidase-deficient fibres and the loss of mitochondrial and nuclear protein subunits indicate the involvement of other, especially nuclear factors.
Similar content being viewed by others
References
Anderson S, Bankier AT, Barrell BG, De Bruin MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Arnaudo E, Dalacas M, Shanske S, Moraes CT, DiMauro S, Schon EA (1991) Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine induced myopathy. Lancet 337:508–510
Attardi G (1981) Organization and expression of the mammalian mitochondrial genome: a lesson in economy. Trends Biochem Sci 6:86–89, 100–103
Attardi G, Schatz G (1988) Biogenesis of mitochondria. Annu Rev Cell Biol 4:289–333
Byrne E, Dennett X, Trounce I, Burdon J (1985) Partial cytochrome oxidase (aa3) deficiency in chronic progressive external ophthalmoplegia — Histochemical and biochemical studies. J Neurol Sci 71:257–271
Byrne E, Dennett X, Trounce I (1991) Oxidative energy failure in postmitotic cells: a major factor in senescence. Rev Neurol 147:532–535
Chomyn A, Mariottini P, Leeter MWJ, Ragan CI, Doolittle RF, Matsuno-Yagi A, Hatefi Y, Attardi G (1985) Functional assignment of the products of the unassigned reading frames of human mitochondrial DNA. In: Quagliariello E, Slater EC, Palmieri F, Saccone C, Kroon AM (eds) Achievements and perspectives of mitochondrial research. vol II Biogenesis, Elsevier, Amsterdam, pp 259–275
Collins S, Rudduck C, Marzuki S, Dennett X, Byrne E (1991) Mitochondrial genome distribution in histochemically cytochrome c oxidase negative muscle fibres in patients with a mixture of deleted and wild type mitochondrial DNA. Biochem Biophys Acta 1097:309–317
Cormier V, Rötig A, Tardieu M, Colonna M, Saudubray JM, Munnich A (1991) Autosomal dominant deletions of the mitochondrial genome in a case of progressive encephalomyopathy. Am J Hum Genet 48:643–648
Cortopassi GA, Arnheim N (1990) Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res 18:6927–6933
Dalacas MC, Illa I, Pezeshkpour, Laukaitis JP, Cohen B, Griffin JL (1990) Mitochondrial myopathy caused by longterm zidovudine therapy. N Engl J Med 322:1098–1105
Degoul F, Nelson I, Lestienne P, Francois D, Romero N, Duboc D, Eymard B, Fardeau M, Ponsot G, Paturneau-Jouas M, Chaussain M, Leroux JP, Marsac C (1991) Deletion of mitochondrial DNA in Kearns-Sayre syndrome and ocular myopathies: genetic biochemical and morphological studies. J Neurol 101:168–177
DiMauro S, Bonilla E, Zeviani M, Nakagawa M, DeVivo DC (1985) Mitochondrial myopathies. Ann Neurol 17:521–538
DiMauro S, Zeviani M, Servidei S, Bonilla E, Miranda AF, Prelle A, Schon EA (1987) Cytochrome c oxidase deficiency: clinical and biochemical heterogeneity. Ann NY Acad Sci 488:19–32
Enter C, Obermaier-Kusser B, Müller-Höcker J, Zierz St, Kurlemann G, Pongratz D, Förster C, Gerbitz KD (1991) A specific point mutation in the mitochondrial genome of Caucasians with MELAS. Hum Genet 88:233–236
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments of high specific activity. Anal Biochem 132:6–13
Fleming JE, Miquel J, Gottroll SF, Yengoyan LS, Economos AC (1982) Is cell ageing caused by respiratory-dependent injury to the mitochondrial genome? Gerontology 28:44–53
Gerbitz KD, Obermaier-Kusser B, Zierz S, Pongratz D, Müller-Höcker J, Lestienne P (1990) Mitochondrial myopathies: divergences of genetic deletions, biochemical defects and the clinical syndromes. J Neurol 237:5–10
Goto Y, Nonoka I, Horai S (1990) A mutation in the tRNA-Leu(UUR)gene associated with the MELAS subgroup of mitochondrial encephalopathies. Nature 348:651–653
Hansford RG (1983) Bioenergetics in ageing. Biochem Biophys Acta 726:41–80
Harding AE (1991) Neurological disease and mitochondrial genes. Trends Neurosci 14:132–138
Harman A (1983) Free radical theory of ageing: consequences of mitochondrial ageing. Age 6:86–94
Hattori K, Tanaka M, Sugiyama S, Obayashi T, Ito T, Satake T, Hanaki Y, Asai Y, Nagano M, Ozawa T (1991) Age dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am Heart J 121:1735–1742
Hayakawa M, Torii K, Sugiyama S, Tanaka M, Ozawa T (1991) Age-assoziated accumulation of 8-hydroxydeoxyguanosine in mitochondrial DNA of human diaphragm. Biochem Biophys Res Commun 179:1023–1029
Hayashi JI, Ohta S, Kikuchi A, Takemitsu M, Goto Y, Nonaka I (1991) Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci USA 88:10614–10618
Holt IJ, Harding AE, Morgan-Hughes JA (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331:717–719
Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, Ozawa T (1990) Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochem Biophys Res Commun 170:1044–1048
Johnson MA, Turnbull DM, Dick DJ, Sherratt HSA (1983) A partial deficiency of cytochrome c oxidase in chronic progressive external ophthalmoplegia. J Neurol Sci 60:31–53
Kadenbach B, Kuhn-Nentwig L, Büge U (1987) Evolution of a regulatory enzyme cytochrome c oxidase (complex IV). Curr Top Bioenerg 15:113–161
Kadenbach B, Müller-Höcker J (1990) Mutations of mitochondrial DNA and human death. Naturwissenschaften 77:221–225
Katayama M, Tanaka M, Yamamoto H, Ohbayashi T, Nimura Y, Ozawa T (1991) Deleted mitochondrial DNA in the skeletal muscle of aged individuals. Biochem Int 25:47–56
Kobayashi Y, Momoi MY, Tominaga K, Momoi T, Nihei K, Yanagisawa M, Kagawa Y, Ohta S (1990) A point mutation in the mitochondrial tRNALeu (UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun 173:816–822
Larsson NG, Holme E, Kristiansson B, Oldfors A, Tulinius M (1990) Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res 28:131–136
Lauber J, Marsac, Kadenbach B, Seibel P (1991) Mutations in mitochondrial tRNA genes: a frequent cause of neuromuscular diseases. Nucleic Acids Res 19:1393–1397
Lestienne P, Ponsot G (1988) Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet I:885
Linnane AW, Marzuki S, Ozawa T, Tanaka M (1989) Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet I:642–645
Linnane AW, Baumer A, Maxwell RJ, Preston H, Zhang C, Marzukis (1990) Mitochondrial gene mutation: the ageing process and degenerative disease. Biochem 22:1067–1076
Lojda Z, Gossrau R, Schiebler TH (1976) Enzymhistochemische Methoden. Springer, Berlin Heidelberg New York
Lombes A, Bonilla E, DiMauro S (1989) Mitochondrial encephalomyopathies. Rev Neurol 145:671–689
Miquel J (1991) An integrated theory of aging as the result of mitochondrial DNA mutation in differentiated cells. Arch Gerontol Geriatr 12:99–117
Miquel J, Economos AC, Fleming J, Johnson JE (1980) Mitochondrial role in cell ageing. Exp Gerontol 15:575–591
Mita S, Schmidt B, Schon EA, DiMauro S, Bonilla E (1989) Detection of “deleted” mitochondrial genomes in cytochrome-c-oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci USA 86:9509–9513
Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S, Nonaka I, Koga Y, Spiro AJ, Brownell KW, Schmidt B, Schotland DL, Zupanc M, DeVivo DC, Schon EA, Rowland LP (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med 320:1293–1299
Moraes CT, Shanske S, Tritschler HJ, Aprille JR, Andreeta F, Bonilla E, Schon EA, DiMauro S (1991) mtDNA depletion with variable tissue expression: a novel abnormality in mitochondrial diseases. Am J Hum Genet 48:492–501
Morgan-Hughes JA, Schapira AHV, Cooper JM, Holt IJ, Harding AE, Clark JB (1990) The molecular pathology of respiratory-chain dysfunction in human mitochondrial myopathies. Biochim Biophys Acta 1018:217–222
Müller-Höcker J (1989) Cytochrome-c-oxidase deficient cardiomyocytes in the human heart. An age-related phenomenon. Am J Pathol 134:1167–1171
Müller-Höcker J (1990) Cytochrome-c-oxidase deficient fibres in the limb muscle and diaphragm of men without muscular disease: an age related alteration. J Neurol Sci 14–21
Müller-Höcker J (1992) Mitochondria and ageing. Brain Pathol 2:149–158
Müller-Höcker J, Schneiderbanger K, Stefani FH, Kadenbach B (1992) Progressive loss of cytochrome-c-oxidase in the extraocular muscles during ageing. A cytochemical-immunocytochemical study. Mutation Res 275:115–124
Müller-Höcker J, Pongratz D, Hübner G (1983) Focal deficiency of cytochrome-c-oxidase in skeletal muscle of patients with progressive external ophthalmoplegia. Virchows Arch (A) 402:61–71
Müller-Höcker J, Stünkel S, Pongratz D, Hübner G (1985) Focal deficiency of cytochrome-c-oxidase and of mitochondrial ATPase with histochemical evidence of loosely coupled oxidative phosphorylation in a mitochondrial myopathy of a patient with bilateral ptosis. An enzyme histochemical, immunohistochemical and fine structural study. J Neurol Sci 69:27–36
Müller-Höcker J, Johannes A, Droste M, Kadenbach B, Pongratz D, Hübner G (1986) Fatal mitochondrial cardiomyopathy in Kearns-Sayre syndrome with deficiency of cytochrome c oxidase in the cardiac and skeletal muscle. An enzymehistochemical-ultraimmunocytochemical fine structural study in longterm frozen autopsy tissue. Virchows Arch [B] 52:353–367
Müller-Höcker J, Droste M, Kadenbach B, Pongratz D, Hübner G (1989) Fatal mitochondrial myopathy with cytochrome-c-oxidase deficiency and subunit restricted reduction of enzyme protein in two siblings. An autopsy-immunocytochemical study. Hum Pathol 20:666–672
Müller-Höcker J, Seibel P, Schneiderbanger K, Zietz C, Obermaier-Kusser B, Gerbitz KD, Kadenbach B (1992) In situ Hybridisation of mitochondrial DNA in the heart of a patient with Kearns-Sayre syndrome and dilatative cardiomyopathy. Hum Pathol (in press)
Nelson I, Degoul F, Obermaier-Kusser B, Romero N, Borrone C, Marsac C, Vayssiére JL, Gerbitz K, Fardeau M, Ponsot G, Lestienne P (1989) Mapping of heteroplasmic mitochondrial DNA deletion in Kearns-Sayre syndrome. Nucleic Acids Res 17:8117–8124
Nohl H (1988) Oxygen radical release in mitochondria: Influence of age. Mod Ageing Res 8:77–99
Obermaier-Kusser B, Müller-Höcker J, Nelson I, Lestienne P, Riedele Th, Gerbitz KD (1990) Detektion of different copy numbers of identically deleted mitochondrial DNA in tissues from a patient with Kearns-Sayre syndrome. Biochem Biophys Res Commun 169:1007–1015
Obermaier-Kusser B, Paetzke-Brunner, Enter C, Müller-Höcker J, Zierz S, Ruitenbeck, Gerbitz KD (1991) Respiratory chain activity in tissues from patients (MELAS) with a point mutation of the mitochondrial genome (tRNALeu(UUR)) FEBS Lett 286:67–70
Otsuka M, Niijima K, Mizuno Y, Yoshida M, Kagawa Y, Ohta S (1990) Marked decrease of mitochondrial DNA with multiple deletions in a patient with familial mitochondrial myopathy. Biochem Biophys Res Com 167:680–685
Petty RKH, Harding AE, Morgan-Hughes JA (1986) The clinical features of mitochondrial myopathy. Brain 109:915–938
Romero NB, Lestienne P, Marsac C, Paturneau-Jouas M, Nelson I, Francois D, Eymard B, Fardeau M (1989) Immunocytological and histochemical correlation in Kearns-Sayre with mtDNA deletion and partial cytochrome c deficiency in skeletal muscle. J Neurol Sci 93:297–309
Schon EA, Rizzuto R, Moraes CT, Nakase H, Zeviani M, Di-Mauro S (1989) A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science 244:346–349
Seibel P, Mell O, Hannemann A, Müller-Höcker J, Kadenbach B (1991) A method for quantitative analysis of deleted mitochondrial DNA by PCR in small tissue samples. Methods Mol Cell Biol 2:147–153
Shanske S, Moraes CT, Lombes A, Miranda AF, Bonilla E, Lewis BA, Whelan HA, Ellsworth CA, DiMauro S (1990) Widespread tissue distribution of mitochondrial DNA deletion in Kearns-Sayre syndrome. Neurology 40:24–28
Shoffner JM, Lott MT, Lezza AMS, Seibel P, Ballinger SW, Wallace DC (1990) Myoclonic epilepsy and ragged-red fibers disease (MERRF) is associated with a mitochondrial DNA tRNAlys mutation. Cell 61:931–937
Shoubridge EA, Karpati G, Hastings KEM (1990) Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fibre segments in mitochondrial disease. Cell 62:43–49
Sugiyama S, Hattori K, Hayakawa M, Ozawa T (1991) Quantitative analysis of age associated accumulation of mitochondrial DNA with deletion in human heart. Biochem Biophys Res Commun 180:894–899
Tanaka M, Ino H, Ohno K, Ohbayashi T, Ikebe S, Sano T, Ichiki T, Kobayashi M, Wada Y, Ozawa T (1991) Mitochondrial DNA mutations in mitochondrial myopathy, encephalopathy lactic acidosis and stroke-like episodes (MELAS). Biochem Biophys Res Commun 174:861–868
Tritschler HJ, Andreeta F, Moraes CT, Bonilla E, Arnaudo E, Danon MJ, Glass S, Zelaya BM, Vamos E, Shanske S, Kadenbach B, DiMauro S, Schon EA (1992) Mitochondrial myopathy of childhood with depletion of mitochondrial DNA. Neurology 42:209–217
Trounce I, Byrne E, Marzuki S (1989) Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet I:637–639
Wallace DC (1989) Mitochondrial DNA mutations and neuromuscular disease. Trends Genet 5:9–13
Yamamoto M, Nonaka I (1988) Skeletal muscle pathology in chronic progressive external ophthalmoplegia with ragged red fibers. Acta Neuropathol 76:558–563
Yen Tch, Su JH, King KL, Wei YH (1991) Ageing associated 5 kb deletion in human liver mitochondrial DNA. Biochem Biophys Res Commun 178:124–131
Yoneda M, Tanno Y, Horai S, Ozawa T, Miyatake T, Tsuji S (1990) A common mitochondrial DNA mutation in the t-RNALys of patients with myoclonus epilepsy associated with ragged-ref fibers. Biochem Int 21:789–796
Yuzaki M, Ohkoshi N, Kanazawa I, Kagawa Y, Ohta S (1989) Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun 164:1352–1357
Zhang C, Baumer A, Maxwell RJ, Linnane AW, Nagley Ph (1992) Multiple mitochondrial DNA deletions in an elderly human individual. FEBS Lett 297:34–38
Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, Rowland LP (1988) Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 38:1339–1346
Zeviani M, Servidei S, Gellera C, Bertini E, DiMauro S, DiDonato S (1989) An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature 339:309–311
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Müller-Höcker, J., Seibel, P., Schneiderbanger, K. et al. Different in situ hybridization patterns of mitochondrial DNA in cytochrome c oxidase-deficient extraocular muscle fibres in the elderly. Vichows Archiv A Pathol Anat 422, 7–15 (1993). https://doi.org/10.1007/BF01605127
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01605127