Abstract
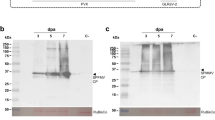
A suspension culture from potato spindle tuber viroid (PSTV)-infected cells of the wild type potato (Solanum demissum) has been established, which is a suitable model system for studying PSTV replicationin vivo. The conditions for rapid growth of these cells and for permanent extensive viroid biosynthesis within them are described. Biosynthesis of PSTV in the potato cells was demonstrated by32P-incorporation into nucleic acids and their subsequent electrophoretic analysis on polyacrylamide gels. Under optimum culture conditions the amount of32P-orthophosphate incorporation into PSTV reached 10% of that incorporated into the 2 M LiCl-soluble cellular RNA. (+)PSTV and its complementary form, i.e. (−)PSTV were identified after their electrophoretic separation on polyacrylamide and agarose gels by molecular hybridization. This analysis revealed the presence of six high molecular weight(−)PSTV species, which are possibly multimers of the unit length(+)PSTV molecule consisting of 359 nucleotides.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- 6BA:
-
6-benzyladenine
- NAA:
-
naphtalene acetic acid
- B5 medium:
-
medium of Gamborget al. (6)
- EDTA:
-
ethylenediaminetetraacetic acid
- LS medium:
-
medium of Linsmaier & Skoog (11)
- Tris:
-
Tris-hydroxymethylaminomethane
- SDS:
-
dodecylsulphate sodium salt
- SSC:
-
0.15 M NaCl/0.015 M Na-citrate
- PCV:
-
packed cell volume
References
Behnke M: Regeneration in Gewebekulturen einiger dihaploider Solanum tuberosum-Klone. Z Pflanzenzüchtg 75: 262–265, 1975.
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976.
Branch AD, Robertson HD, Dickson EC: Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci USA 78:6381–6385, 1981.
Commerford SL: Iodination of nucleic acids in vitro. Biochemistry 10:1993–1999, 1971.
Diener TO: Potato spindle tuber ‘virus’. IV. A replicating, low molecular weight RNA. Virology 45:411–428, 1971.
Gamborg OL, Miller RA, Ojima K: Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158, 1968.
Gilbert W, Dressler D: DNA-replication: The rolling circle model. Cold Spring Harbor Symp Quant Biol 33:473–484, 1980.
Goldman D, Merril CR: Silver staining of DNA in polyacrylamide gels: linearity and effect of fragment size. Electrophoresis 1:24–26, 1982.
Grill LK, Semancik JS: RNA sequences complementary to citrus exocortis viroid in nucleic acid preparations from infected Gynura aurantiaca. Proc Natl Acad Sci USA 75:896–900, 1978.
Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sänger HL: Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 273:203–208, 1978.
Linsmaier EM, Skoog F: Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127, 1968.
McMaster GK, Carmichael GG: Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA 74:4835–4838, 1977.
Mühlbach HP, Sänger HL: Multiplication of cucumber pale fruit viroid in inoculated tomato leaf protoplasts. J Gen Virol 35:377–386, 1977.
Mühlbach HP, Sänger HL: Viroid replication is inhibited by alpha-amanitine. Nature 278:185–188, 1979.
Mühlbach HP, Sänger HL: Continuous replication of potato spindle tuber viroid in permanent cell cultures of potato and tomato. Biosci Rep 1:79–87, 1981.
Owens RA, Diener TO: RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci USA 79:113–117, 1982.
Rackwitz HR, Rohde W, Sänger HL: DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature 291:297–301, 1981.
Rohde W, Sänger HL: Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci Rep 1:327–336, 1981.
Sänger HL: Biology, structure, functions and possible origin of viroids. In: Parthier B, Boulter D (eds) Encyclopedia of Plant Physiology, New Series, Vol 14B, Nucleic Acids and Proteins in Plants II, Springer Berlin, Heidelberg, New York, 1982, pp 368–454.
Sänger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK: Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA 73:3852–3856, 1976.
Sänger HL, Ramm K: Radioactive labelling of viroid RNA. In: Markham R, Davies DR, Hopwood DA, Horne RW (eds) Modification of the Information Content of Plant Cells. North-Holland, Amsterdam, 1975, pp 229–252.
Sänger HL, Ramm K, Domdey H, Gross HJ, Henco K, Riesner D: Conversion of circular viroid molecules to linear strands. FEBS Lett 99:117–122, 1979.
Stellwag EJ, Dahlberg AE: Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM)-paper. Nucleic Acids Res 8:299–317, 1980.
Wahl GM, Stern M, Stark GR: Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization using dextran sulfate. Proc Natl Acad Sci USA 76:3683–3687, 1979.
Zelcer A, Van Adelsberg J, Leonard DA, Zaitlin M: Plant cell suspension cultures sustain long-term replication of potato spindle tuber viroid. Virology 109:314–322, 1982.
Zelcer A, Zaitlin M, Robertson HD, Dickson E: Potato spindle tuber viroid-infected tissues contain RNA complementary to the entire viroid. J Gen Virol 59:139–148, 1982.
Author information
Authors and Affiliations
Additional information
The infectious and sequenced PSTV is arbitrarily designated (+)PSTV
Rights and permissions
About this article
Cite this article
Mühlbach, HP., Faustmann, O. & Sänger, H.L. Contitions for optimal growth of a PSTV-infected potato cell suspension and detection of viroid-complementary longer-than-unit-length RNA in these cells. Plant Mol Biol 2, 239–247 (1983). https://doi.org/10.1007/BF01578642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01578642