Summary
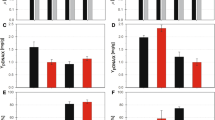
Four recombinant strains ofEscherichia coli were examined for the effects of the dissolved oxygen level on the level of biomass, the plasmid content, and the level of recombinant protein at the stationary phase of batch growth. Strains JM101/pYEJ001, and TB-1/pYEJ001 (encoding chloramphenicol acetyltransferase), and strain TB-1/p1034, and TB-1/pUC19 (encoding β-galactosidase) were grown at the constant dissolved oxygen levels of 0, 50, and 100% air saturation, as well as in the absence of dissolved, oxygen control. The biomass of all strains under constant aerobic conditions was 12–36 times higher than that under anaerobic conditions, but was the same as or slightly higher than that without dissolved oxygen control. The plasmid content in all strains under anaerobic conditions was 2.9–11.7 times higher than that under aerobic conditions. The optimal dissolved oxygen concentration for the specific activity of recombinant proteins was dependent upon the strain. In no strain were constant aerobic conditions optimal. However, because of the effect on biomass, controlled aerobic conditions were optimal for the volumetric activity of recombinant protein in all but one strain.
Similar content being viewed by others
References
Arcuri, E.J., K. Turner, D. Sharr and B. Okita. 1988. The influence of dissolved oxygen limitation upon the accumulation of heterogeneous proteins inEscherichia coli. SIM News 4: 33.
Bailey, F.J., J. Blankenship, J.H. Condra, R.Z. Maigetter and R.W. Ellis. 1987. High-cell-density fermentation studies of a recombinantEscherichia coli that expresses atrial natriuretic factor. J. Ind. Microbiol. 2: 47–52.
Brauer, H. 1985. In: Biotechnology (Brauer, H., ed.), pp. 161, VCH, Weinheim, F.R.G.
Bauer, S. and E. Zin. 1976. Dense growth of aerobic bacteria in a bench-scale fermentor. Biotechnol. Bioeng. 18: 81–94.
Clark, T.A., T. Hesketh and T. Seddon. 1985. Automatic control of dissolved oxygen tension via fermentor agitation speed. Biotechnol. Bioeng. 27: 1507.
Chambers, S.P., S.E. Prior, D.A. Bartow and N.P. Minton. 1988. The pMTL nic− cloning vectors. Gene 68: 139–149.
Cutayar, J.M. and D. Poillon. 1989. High cell density culture ofE. coli in a fed-batch system with dissolved oxygen as a substrate feed indicaton. Biotechnol. Lett. 11: 155–160.
Doelle, H.W. 1981. In: Biotechnology (Rehm, H.-J. and G. Reed, eds.), pp. 196, Verlag Chemie, Weinheim, F.R.G.
Epstein, W., L.B. Rothman-Denes and J. Hesse. 1975. Adenosine 3′, 5′-cyclic monophosphate as mediator of catabolic repression inEscherichia coli. Proc. Natl. Acad. Sci. USA 72: 2300.
Flynn, D.S. and M.D. Lilly. 1967. A model for the control of the dissolved oxygen tension in microbial cultures. Biotechnol. Bioeng. 9: 515–531.
Gleiser, I.E. and S. Bauer. 1981. Growth ofE. coli W to high cell density by oxygen level linked control of carbon source concentration. Biotechnol. Bioeng. 23: 1015.
Goldberg, A.L. and S.A. Goff. 1986. The selective degradation of abnormal proteins in bacteria. In: Maximizing Gene Expression (Reznicoff, W. and L. Gold, eds.), pp. 287–314, Butterworth Publishers, Stoneham.
Hopkins, D.J., M.J. Betenbaugh and P. Dhurjati. 1987. Effects of dissolved oxygen shock on the stability of recombinantEscherichia coli containing plasmid pKN401. Biotechnol. Bioeng. 29: 85–91.
Koizumi, J., Y. Monden and S. Aiba. 1985. Effects of temperature and dilution rate on the copy number of recombinant plasmid in continuous culture ofBacillus stearothermophilus (pLP11). Biotechnol. Bioeng. 27: 721–728.
Lancaster, M.J., R.J. Sharp, J.R. Court, I.D. McEntee, R.G. Melton and R. Sherwood. 1989. Production of cloned carboxypeptidase G2 byEscherichia coli: genetic and environmental considerations. Biotechnol. Lett. 10: 699–704.
Laemmli, U.K. 1970. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680.
Lee, G.M., K.B. Son, S.K. Rhee and M.H., Han. 1986. Plasmid maintenance and growth of recombinantSaccharomyces cerevisiae producing hepatitis B virus surface antigen. Biotechnol. Lett. 8: 385–390.
Lee, Y.L. and H.N. Chang. 1988. High cell density continuous culture ofEscherichia coli producing penicillin acylase. Biotechnol. Lett. 10: 787–792.
Li, X., J.W. Robbins, Jr. and K.B. Taylor. 1990. The production of recombinant beta-galactosidase inEscherichia coli in yeast extract enriched medium. J. Ind. Microbiol. 5: 85–94.
Maniatis, T., E.F. Fritsch and J. Sambrook. 1982. Transformation ofEscherichia coli by plasmid DNA. In: Molecular Cloning, pp. 249–255, Cold Spring Harbor Laboratory, New York, NY.
Maniatis, T., E.F. Frisch and J. Sambrook. 1982. Rapid isolation of plasmid or bacteriophage DNA. In: Molecular Cloning, pp. 365–373, Cold Spring Harbor Laboratory, New York, NY.
Mizukami, T., Y. Komatsu, N. Hosoi, S. Itoh and T. Oka. 1986. Production of active human interferon-B inEscherichia coli. Biotechnol. Lett. 9: 605–610.
Robbins, J.W., Jr. and K.B. Taylor. 1989. Optimization ofEscherichia coli growth by controlled addition of glucose. Biotechnol. Bioeng. 34: 1289–1294.
Rollins, M.J., S.E. Jensen and D.W.S. Westlake. 1988. Effect of aeration of antibiotic production byStreptomyces clavuligerus. J. Ind. Microbiol. 3: 357–364.
Ryan, W., S.J. Parulekar and B.C. Stark. 1989. Expression of B-lactamase by recombinantEscherichia coli strains containing plasmids of different sizes—effects of pH, phosphate, and dissolved oxygen. Biotechnol. Bioeng. 34: 309–319.
Seo, J.-H. and J.E. Bailey. 1985. Effects of recombinant plasmid content on growth properties and cloned gene product formation inEscherichia coli. Biotechnol. Bioeng. 27: 1668–1674.
Shaw, W.V. and R.F. Brodsky. 1968. Characterization of chloramphenicol acetyltransferase from chloramphenicol resistantStaphylococcus aureus. J. Bacteriol 95: 28–36.
Stainer, R.Y., J.L. Ingraham, M.L. Wheelis and P.R. Painter. 1986. In: The Microbial World, pp. 210, Prentice-Hall, New York.
Tolentino, G.J. and K.-Y. San. 1988. Plasmid maintenance and gene expression of a recombinant culture under aerobic and anaerobic conditions. Biotechnol. Lett. 10: 373–376.
Wang, D.I.C., C.L. Cooney, A.L. Demain, P. Dunnill, A.E. Humphrey and M.M. Lilly. 1978. In: Fermentation and Enzyme Technology, pp. 91, Wiley, New York.
Weber, A.E. and K.-Y. San. 1987. Presistence and expression of the plasmid pBR322 inEscherichia coli K-12 cultured in complex medium. Biotechnol. Lett. 11: 757–760.
Yegneswaran, P.K., M.R. Gray and D.W.S. Westlake. 1988. Effects of reduced oxygen on growth and antibiotic production inStreptomyces clavuligerus. Biotechnol. Lett. 10: 479–484.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, X., Robbins, J.W. & Taylor, K.B. Effect of the levels of dissolved oxygen on the expression of recombinant proteins in four recombinantEscherichia coli strains. Journal of Industrial Microbiology 9, 1–9 (1992). https://doi.org/10.1007/BF01576362
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01576362