Abstract
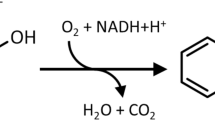
The genes encoding aniline utilization inPseudomonas sp. strain CIT1 have been cloned inEscherichia coli and partially characterized. Molecular cloning of the genes was achieved by construction of a cosmid library, followed by mobilization of the library into mutants ofPs. sp. CIT1 impaired in a number of functions necessary for growth on aniline. A 42-kbSau3A fragment was found to encode the ability to utilize aniline and contained the catechol 2,3-dioxygenase (C230) gene. The regions encoding these activities were subcloned and further characterized. Plasmids containing the aniline oxidase gene encoded a 260-kDa protein complex, which was putatively shown to be composed of 72 kDa and possibly 36 kDa subunits. The fragment required for C230 activity encodes a 35 kDa protein, similar in size to C230 gene products previously characterized.
Similar content being viewed by others
Literature Cited
Anson JG, Mackinnon G (1984) A novelPseudomonas plasmid involved in aniline degradation. Appl Environ Microbiol 48:868–869
Bachofer R, Lingens F, Schafer W (1975) Conversion of aniline into pyrocatechol by aNocardia sp.: Incorporation of oxygen-18. FEBS Lett 50:288–290
Clewell DB, Helinski DR (1969) Supercoiled circular DNA-protein complexes inEscherichia coli: purification and induced conversion to an open circular form. Proc Natl Acad Sci USA 62:1159–1166
Collins J (1979) Cell free synthesis of proteins coding for mobilisation functions of pCo1E1 and transposition functions of Tn3. Gene 6:29–42
Dagert M, Ehrlich SD (1979) Prolonged incubation in calcium chloride improves the competence ofEscherichia coli cells. Gene 6:23–28
Dagley S, Stopher DA (1959) A new mode of fission of the benzene nucleus by bacteria. Biochem J 73:16p
Davis G, Hill HAO, Aston WJ, Higgins IJ, Turner APF (1983) Bioelectrochemical fuel cell and sensor based on a quinoprotein, alcohol dehydrogenase. Enzyme Microb Technol 5:383–388
Devries JK, Zubay G (1967) DNA-directed peptide synthesis II. The synthesis of the alpha-fragment of the enzyme ß-galactosidase. Proc Natl Acad Sci USA 57:1010–1012
Dymock D (1988) Studies on the conjugation system of the Inc-W plasmid pSa. Ph.D Thesis, Cranfield Institute of Technology.
Erickson MD, Pelizzari F (1977) Identification of polychlorinated biphenyls and other related chemicals in municipal sewage sludge. Washington, DC: Environ Protect Agency 560/6-77-021
Franklin FCH, Bagdasarian M, Bagdasarian MM, Timmis KN (1981) Molecular and functional analysis of the TOL plasmid pWWO fromPseudomonas putida and cloning of genes for the entire regulated aromatic ringmeta cleavage pathway. Proc Natl Acad Sci USA 78:7458–7462
Frey J, Bagdasarian M, Feiss D, Franklin FCH, Deshusses J (1983) Stable cosmid vectors that enable the introduction of cloned fragments into a wide range of Gram negative bacteria. Gene 62:237–247
Hantke K (1981) Regulation of ferric iron transport inEscherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet 182:288–292
Hepstinall J, Quayle JR (1970) Pathways leading to and from serine during growth ofPseudomonas AM1 on C1 compounds or succinate. Biochem. J 117:563–572
Hogrefe C, Friedrich B (1984) Isolation and characterisation of megaplasmid DNA from lithoautotrophic bacteria. Plasmid 12:161–169
Kearney PC, Plimmer JW (1972) Metabolism of 3,4 dichloroaniline in soils. J Agric Food Chem 20:584–585
Keil H, Lebens MR, Williams PA (1985) TOL plasmid pWW15 contains two non-homologous independently regulated catechol 2,3 dioxygenase genes. J Bacteriol 163:248–255
Kilby BA (1948) The bacterial oxidation of phenol to β-ketoadipate. Biochem J 43:v.
Maniatis T, Fritsch EF, Sambrooke F (1982) Molecular cloning: A laboratory handbook, 1st edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory.
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Meyers NL (1990) Genetic aspects of aniline degradation inPseudomonas sp. strain CIT1. Ph.D Thesis, Cranfield Institute of Technology.
Rhodes ME (1970) Aniline utilisation byAlcaligenes faecalis: a taxonomic reappraisal. J Appl Bacteriol 33:714–720
Saint CP, McClure NC, Venables WA (1990) Physical map of the aromatic amine andm-toluate catabolic plasmid pTDN1 inPseudomonas putida: location of a uniquemeta cleavage pathway. J Gen Microbiol 136:615–625
Simon R, Priefer U, Puhler A (1983) A broad host range mobilisation system forin vivo genetic engineering: Transposon mutagenesis in Gram negative bacteria. Bio/technol 1:784–791
Sprott GD, Corke CT (1971) Formation of 3,3,4,4', tetrachloroazobenzene from 3,4 dichloroaniline in Ontario soils. Can J Microbiol 17:235–239
Surovtseva EG, Vol'nova AI (1980) Aniline as a source of carbon, nitrogen and energy forAlcaligenes faecalis. Mikrobiologiya 49:49–53
Weiss AA, Hewlet EL, Myers GA, Falkow S (1983) Tn5 induced mutations affecting virulence factors ofBordetella pertussis. J Infect Immun 42:33–41
Wheatcroft R, Williams PA (1981) Rapid methods for the study of both stable and unstable plasmids inPseudomonas. J Gen Microbiol 124:433–437
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meyers, N.L. Molecular cloning and partial characterization of the pathway for aniline degradation inPseudomonas sp. strain CIT1. Current Microbiology 24, 303–310 (1992). https://doi.org/10.1007/BF01571099
Issue Date:
DOI: https://doi.org/10.1007/BF01571099