Abstract
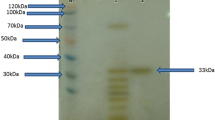
Bacterial isolates from the gland of Deshayes of the marine shipworm (Psiloteredo healdi) produced extracellular protease activity when cultured with 1% cellulose. A protease with a relative molecular mass of 36,000 daltons as determined by SDS-PAGE and a pI of 8.6 was isolated from the medium and purified to electrophoretic homogeneity. No carbohydrate appeared to be associated with the protein. The enzyme was activated and stabilized by relatively high salt concentrations (>0.2M). Below 0.1M salt, significant protein aggregation occurred, as well as autohydrolysis of the protease, both of which resulted in the loss of activity. The specific activity of the enzyme was 65,840 proteolytic units/mg with azocasein substrate of optimal temperature (42°C), pH (9.0), and salt concentration (0.20M NaCl). The activity was stable up to 40°C, from pH 3.0 to pH 11.9, and from 0.1M to 3.5M NaCl. These stabilities, as well as the protease's stability in the presence of chelators, oxidizing agents, and heavy metals, suggest the enzyme has potential for use in relatively low temperature (40°C) industrial applications.
Similar content being viewed by others
Literature Cited
Austin B (1989) Novel pharmaceutical compounds from marine bacteria. J Appl Bacteriol 67:461–470
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brakke MK (1963) Photometric scanning of centrifuged density gradient columns. Anal Biochem 5:271–283
Cotta MA, Hespell RB (1986) Proteolytic activity of the ruminal bacteriumButyrivibrio fibrisolvens. Appl Environ Microbiol 52:51–58
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determining sugars and related substances. Anal Chem 28:350–356
Edman P, Begg G (1967) A protein sequentor. Eur J Biochem 1:80–91
Gianazza E, Righetti PG (1979) Facts and artifacts in isoelectric focusing. In: Redola BJ (ed) Electrophoresis. Berlin, New York: Walter de, Gruyter, pp 129–140
Grant WD, Mwatha WE, Jones BE (1990) Alkaliphiles: ecology, diversity and applications. FEMS Microbiol Rev 75:255–270
Greene RV, Freer SN (1986) Growth characteristics of a novel nitrogen-fixing cellulolytic bacterium. Appl Environ Microbiol, 52:982–986
Greene RV, Griffin HL, Freer SN (1988) Purification and characterization of an extracellular endoglucanase from the marine shipworm bacterium. Arch Biochem Biophys 267:334–341
Greene RV, Cotta MA, Griffin HL (1990) A novel, symbiotic bacterium isolated from marine shipworm secretes proteolytic activity. Curr Microbiol 19:353–356
Griffin HL, Freer SN, Greene RV (1987) Extracellular endoglucanase activity by a novel bacterium isolated from marine shipworm. Biochem Biophys Res Commun 144:143–151
Hewick RM, Hunkapiller MW, Hood LE, Dryer WJ (1981) A gas-liquid solid phase peptide and protein sequentor. J Biol Chem 256:7990–7997
Kirk RE, Othmer DE (1980) Enzyme detergents and industrial enzymes. In: Kirk-Ozhzaer encyclopedia of chemical technology, 3rd edn, Vol. 9. New York: John Wiley & Sons, pp 138–224
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Martin RG, Ames BN (1961) A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem 236:1372–1379
Saravani G-A, Cowan DA, Daniel RM, Morgan HW (1989) Caldolase, a chelator-insensitive extracellular serine proteinase from aThermus spp. Biochem J 262:409–416
Scheuer PJ (1990) Some marine ecological phenomena: chemical basis and biomedical potential. Science 248:173–177
Waterbury JB, Calloway CB, Turner RD (1983) A cellulolytic nitrogen-fixing bacterium cultured from the gland of Deshayes in shipworm (Bivalvia: Teredinidae) Science 221:1401–1403
Waterbury JB, Calloway CB, Turner RD (1989) Bacteria for cellulose digestion. U.S. Patent Number 4,861,721
Whitaker JR (1972) Principles of enzymology for the food sciences. New York: Marcel Dekker, Inc
Williams KW, Soderberg L (1979) A carrier ampholyte for isoelectric focusing. International Laboratory, Jan/Feb
Zaborsky OR, Attaway DH, Mitsui A (1990) Japanese marine biotechnology: new opportunities for industrial microbiology. SIM News 40:45–51
Author information
Authors and Affiliations
Additional information
The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned.
Rights and permissions
About this article
Cite this article
Griffin, H.L., Greene, R.V. & Cotta, M.A. Isolation and characterization of an alkaline protease from the marine shipworm bacterium. Current Microbiology 24, 111–117 (1992). https://doi.org/10.1007/BF01570907
Issue Date:
DOI: https://doi.org/10.1007/BF01570907