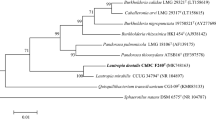
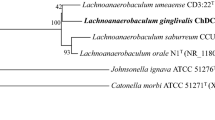
Abstract
A new coccobacillary, nonmotile, Gram-positive, methane-producing organism was isolated from human subgingival plaque. Both hydrogen and carbon dioxide were required for growth. No methane was produced from acetate, formate, or methanol. The optimum pH was 6.9–7.4, and the optimum temperature was 36–38°C. Fecal extract was required for growth, and a volatile fatty acid mixture was highly stimulatory. The DNA G+C content was 28 mol%. On the basis of these characteristics, DNA-DNA hybridization studies, and electrophoretic analysis of cellular proteins, the isolate was considered a new species and namedMethanobrevibacter oralis.
Similar content being viewed by others
Literature Cited
Balch WE, Fox GE, Magnum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluations of a unique biological group. Microbiol Rev 43:260–296
Barnes EM, Impey CS (1974) The occurrence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bacteriol 37:393–409
Belay N, Johnson R, Rajagopal BS, Conway de Macario E, Daniels L (1988) Methanogenic bacteria from human dental plaque. Appl Environ Microbiol 54:600–603
Biavati B, Castagnoli P, Trovatelli LD (1986) Species of the genusBifidobacterium in the feces of human adults. Microbiologica 9:39–45
Boone RD, Mah RA (1989) Methanogenic archaebacteria. In: Stanley JT, Bryant MP, Pfenning N, Holt JG (eds) Bergey's manual of systematic bacteriology. Baltimore: Williams and Wilkins, pp 2173–2216
Boone DR, Whitmann WB (1988) Proposal of minimal standard describing new taxa of methanogenic bacteria. Int J Syst Bacteriol 38:212–219
Brusa T, Conca R, Ferrara A, Ferrari A, Pecchioni A (1987) The presence of methanobacteria in human subgingival plaque. J Clin Periodontol 14:470–471
Brusa T, Canzi E, Allievi L, Del Puppo E, Ferrari A (1993) Methanogens in the human intestinal tract and oral cavity. Curr Microbiol 27:261–265
DSM—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (1989) Catalogue of strains, 4th ed, p 289
Holdeman LV, Moore WEC (1973) Anaerobic laboratory manual, 4th ed., Virginia Polytechnic Institute and State University Anaerobe Laboratory
Johnson JI (1985) DNA reassociation and RNA hybridization of bacterial nucleic acids. In: Gottschalk G (ed) Methods in microbiology, vol 18. New York: Academic Press, pp 33–74
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Miller TL, Wolin MJ (1974) A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol 27:985–987
Miller TL, Wolin MJ (1982) Enumeration ofMethanobrevibacter smithii in human faeces. Arch Microbiol 131:14–18
Miller TL, Wolin MJ (1985)Methanosphera stadmaniae gen. nov. sp. nov.; a species that forms methane by reducing methanol with hydrogen. Arch Microbiol 141:116–122
Miller TL, Wolin MJ, Conway de Macario E, Macario AJL (1982) Isolation ofMethanobrevibacter smithii from human feces. Appl Environ Microbiol 43:227–232
Moore WEC, Hasch DE, Holdeman LV, Cato, EP (1980) Polyacrylamide slab gel elecrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol 39:900–907
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Smith PH, Hungate RE (1958) Isolation and characterization ofMethanobacterium ruminantium n. sp. J Bacteriol 75:713–718
Socransky SS, Gibson RJ, Dale AC, Bortmich L, Rosental E, MacDonald JB (1963) The microbiota of the gingival crevice area of man. Arch Oral Biol 8:275–280
Taylor CD, McBride BC, Wolfe RS, Bryant MP (1974) Coenzyme M, essential for growth of a rumen strain ofMethanobacterium ruminantium. J Bacteriol 120:974–975
Zeikus JG, Bowen VG (1975) Comparative ultrastructure of methanogenic bacteria. Can J Microbiol 21:121–129
Zeikus JG, Henning DL (1975)Methanobacterium arboriphilicum sp. nov., an obligate anaerobe isolated from wetwood of living trees. Antonie van Leeuwenhoek 41:543–552