Abstract
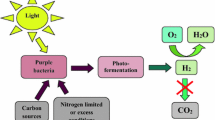
Chlorate or trimethylamine-N-oxide (TMAO) added to phototrophic cultures ofRhodobacter sphaeroides DSM 158 increased both the growth rate and the growth yield although this stimulation was not observed in the presence of tungstate. This strain, exhibited basal activities of nitrate, chlorate, and TMAO reductases independently of the presence of these substrates in the culture medium, and nitrate reductase (NR) activity was competitively inhibited by chlorate. Phototrophic growth ofRhodobacter capsulatus B10, a strain devoid of NR activity, was inhibited only by 100 mM chlorate. However, growth of the nitrate-assimilatingR. capsulatus strains E1F1 and AD2 was sensitive to 10mm chlorate, and their NR activities were not inhibited by chlorate. Both NR and chlorate reductase (CR) activities of strain E1F1 were induced in the presence of nitrate or chlorate respectively, whereas strain AD2 showed basal levels of these activities in the absence of the substrates. A basal TMAO reductase (TR) activity was also observed when these strains ofR. capsulatus were cultured in the absence of this electron acceptor. These results suggest that chlorate and TMAO can be used as ancillary oxidants byRhodobacter strains and that a single enzyme could be responsible for nitrate and chlorate reduction inR. sphaeroides DSM 158, whereas these reactions are catalyzed by two different enzymes inR. capsulatus E1F1 and AD2.
Similar content being viewed by others
Literature Cited
Alef K (1987) The interaction between dimethylsulfoxide and nitrate reducing pathways inRhodobacter capsulatus. FEMS Microbiol Lett 48:11–14
Bisen PS, Shanthy S (1992) Physiological and biochemical characterization of chlorate-resistant mutants ofAnabaena doliolum. Curr Microbiol 25:353–357
Byrne MD, Nicholas DJD (1987) A membrane-bound dissimilatory nitrate reductase fromRhodobacter sphaeroides f.sp.denitrificans. Biochim biophys Acta 915:120–124
Castillo F, Caballero FJ, Cárdenas J (1981) Nitrate photoassimilation by the phototrophic bacteriumRhodopseudomonas capsulata E1F1. Z Naturforsch 36:1025–1029
Cawse PA (1967) The determination of nitrate in soil solution by ultraviolet spectrophotometry. Analyst 92:311–315
Deane-Drummond CE (1984) Nitrate transport intoCharacorallina cells using36ClO3 − as an analogue for nitrate. I. Interaction between36ClO3 − and NO3 −, and characterization of36ClO3 −/NO3 −-influx. J Exp Bot 35:1289–1298
De Groot GN, Stouthamer AH (1969) Regulation of reductase formation inProteus mirabilis. I. Formation of reductases and enzymes of the formic hydrogenlyase complex in the wild type and in chlorate resistant mutants. Arch Microbiol 66:220–233
Doddema H, Telkamp GP (1979) Uptake of nitrate by mutants ofArabidopsis thaliana disturbed in uptake or reduction of nitrate. Physiol Plant 45:332–338
Ferguson SJ, Jackson JB, McEwan AG (1987) Anaerobic respiration in theRhodospirillaceae characterisation of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol Rev 46:117–143
Kelly DJ, Richardson DJ, Ferguson SJ, Jackson JB (1988) Isolation of transposon Tn5 insertion, mutants ofRhodobacter capsulatus unable to reduce trimethylamine-N-oxide and dimethylsulphoxide. Arch Microbiol 150:138–144
Kerber NL, Cárdenas J (1982) Nitrate reductase fromRhodopseudomonas sphaeroides. J Bacteriol 150:1091–1097
Lowry OH, Rosebrough MJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Malmqvist A, Welander T, Gunnarsson L (1991) Anaerobic growth of microorganisms with chlorate as an electron acceptor. Appl Environ Microbiol 57:2229–2232
Martínez-Luque M, Dobao MM, Castillo F (1991) Characterization of the assimilatory and dissimilatory nitrate-reducing systems inRhodobacter: a comparative study. FEMS Microbiol Lett 83:329–334
McEwan AG, Cotton NPJ, Ferguson SJ, Jackson JB (1985) The role of auxiliary oxidants in the maintenance of a balanced redox poise for photosynthesis in bacteria. Biochim Biophys Acta 810:140–147
McEwan AG, Wetzstein HG, Meyer O, Jackson JB, Ferguson SJ (1987) The periplasmic nitrate reductase ofRhodobacter capsulatus: purification, characterisation and distinction from a single reductase for trimethylamine-N-oxide, dimethylsulphoxide and chlorate. Arch Microbiol 147:340–345
Moreno-Vivián C, Cárdenas J, Castillo F (1986)In vivo short-term inhibition of nitrogenase by nitrate inRhodopseudomonas capsulata E1F1. FEMS Microbiol Lett 34:105–109
Nakagawa H, Yamashita N (1986) Chlorate reducing activity of spinach nitrate reductase. Agric Biol Chem 50:1893–1894
Pichinoty F (1966) Propriétés, régulation et fonctions physiologiques des nitrate-réductase bacteriennes A et B Bull Soc Fran Physiol Vég 12:97–104
Pino-Pérez F, Pérez-Bendito D (1983) Análisis des los elementos-traza por espectrofotometría de absorción molecular UV-visible. Córdoba: Universidad de Sevilla y Monte de Piedad y Caja de Ahorros de Córdoba, pp 421–423
Prieto R, Fernández E (1993) Toxicity of and mutagenesis by chlorate are independent of nitrate reductase activity inChlamydomonas reinhardtii. Mol Gen Genet 237:429–438
Snell FD, Snell CT (1949) Colorimetric methods of analysis, 3rd ed, vol 2., Princeton, N.J.: D. van Nostrand Reinhold, pp 804–805
Solomonson LP, Vennesland B (1972) Nitrate reductase and chlorate toxicity inChlorella vulgaris Beijerinck. Plant Physiol 50:421–424
Weaver PF, Wall JD, Gest H (1975) Characterization ofRhodopseudomonas capsulata. Arch Microbiol 105:207–216
Willison JC (1990) Derivative ofRhdobacter capsulatus strain AD2 cured of their endogenous plasmid are unable to utilize nitrate. FEMS Microbiol Lett 66:23–28
Witt A, Klemme JH (1991) No correlation between plasmid content and ability to reduce nitrate in wild-type strains ofRhodobacter capsulatus. Z Naturforsch 46:703–705
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roldán, M.D., Reyes, F., Moreno-Vivián, C. et al. Chlorate and nitrate reduction in the phototrophic bacteriaRhodobacter capsulatus andRhodobacter sphaeroides . Current Microbiology 29, 241–245 (1994). https://doi.org/10.1007/BF01570161
Issue Date:
DOI: https://doi.org/10.1007/BF01570161