Abstract
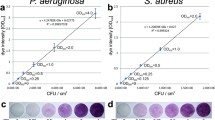
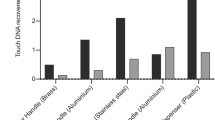
This paper describes a technique which reproducibly quantifies the ease of removal of microorganisms from surfaces. Tiles (22 mm×22 mm) of various materials were colonised withStaphylococcus epidermidis NCTC 11047,Escherichia coli K12 HB101 orPseudomonas aeruginosa PaWH, by submersion, for various times (2 min–48 h), in inoculated Tryptone Soya broth (37°C). Colonised tiles were blotted onto a Tryptone Soya agar plate for 1 min and the process was repeated through a succession of agar plates. The final plate contained tetrazolium salts (0.05% w/v) and was incubatedin situ with the tile. Tetrazolium plates indicated that very few organisms remained on the tiles after 15 successive blots. In all instances, the number of recovered colonies per plate decreased exponentially with plate succession number, according to the relationship, CFU-A.10−kN, where CFU is the number of colonies transferred,k is the removal exponent, A is the intercept and N is the plate succession number. Removal exponents differed significantly between organisms (P>0.95), depended on the nature of the test surface, and decreased as the inital attachment and colonisation time was increased from 2 min–48 h. Intercept values (A) but not the gradients were dependent upon the initial numbers of bacteria in suspension. These data indicate that the gradients derived from counting recoverable viable cells from successive blots of test tiles onto agar is a measure of the strength of attachment of the organisms to the surface.
Similar content being viewed by others
References
Allison DG, MRW Brown, DJ Evans and P Gilbert. 1990. Surface hydrophobicity and dispersal ofPseudomonas aeruginosa from biofilms. FEMS Microbiol Lett 71: 101–104.
Allison DG, DJ Evans, MRW Brown and P Gilbert. 1990. Possible involvement of the division cycle in dispersal ofEscherichia coli from biofilms. J Bacteriol 172: 1667–1669.
Anwar H, MK Dasgupta, K Lam and JW Costerton. 1989. Tobramycin resistance of mucoidPseudomonas aeruginosa biofilm grown under iron-limitation. J Antimicrob Chemother 24: 647–655.
Anwar H, JL Strap and JW Costerton. 1992. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial chemotherapy. Antimicrob Agents Chemother 36: 1347–1351.
Brown MRW, DG Allison and P Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth rate related effect. J Antimicrob Chemother 22: 777–789.
Costerton JW, RT Irvin and KJ Cheng. 1981. The bacterial glycocalyx in nature and disease. Ann Rev Microbiol 35: 399–424.
Costerton JW and ES Lashen. 1984. Influence of biofilm on efficacy of biocides on corrosion-causing bacteria. Mat Perform 9: 13–17.
Costerton JW, KJ Cheng, GG Geesy, TI Ladd, JC Nickel, M Dasgupta and TJ Marrie. 1987. Bacterial biofilms in nature and disease. Ann Rev Microbiol 41: 435–464.
Dagostino L, AE Goodman and KC Marshall. 1991. Physiological responses induced in bacteria adhering to surfaces. Biofouling 4: 113–119.
Evans E, MRW Brown and P Gilbert. 1994. Iron chelator, exopolysaccharide and protease production onStaphylococcus epidermidis: a comparative study of the effects of specific growth rate in biofilm and planktonic culture. Microbiology 140: 153–157.
Fletcher M. 1984. Comparative physiology of attached and free-living bacteria. In: Microbial Adhesion and Aggregation (Marshall KC, ed), pp 223–232, Springer-Verlag, Berlin.
Gambello MJ, S Kaye and BH Iglewski. 1993.LasR ofPseudomonas aeruginosa is a transcriptional activator of the line protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun 61: 1180–1184.
Gibbons RJ and J van Houte. 1975. Bacterial adherence in oral microbial ecology. Ann Rev Microbiol 29: 19–44.
Gilbert P, PJ Collier and MRW Brown. 1990. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle and dormancy. Antimicrob Agents Chemother 34: 1865–1868.
Gilbert P, DJ Evans, E Evans, IG Duguid and MRW Brown. 1991. Surface characteristics and adhesion ofEscherichia coli andStaphylococcus epidermidis in batch culture. J Appl Bacteriol 71: 72–77.
Giwercman B, ET Jensen, N Hoiby, A Kharazmi and JW Costerton. 1991. Induction of β-lactamase production inPseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 35: 1008–1010.
Holah JT, SF Bloomfield, AJ Walker and H Spenceley. 1994. Control of biofilms in the food industry. In: Bacterial Biofilms and Their Control in Medicine and Industry (Wimpenny J, W Nichols, D Stickler and H Lappin-Scott, eds), pp 163–168, Bioline Press, Cardiff.
Hoyle BD, J Jass and JW Costerton. 1990. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother 26: 1–6.
Lappin-Scott HM and JW Costerton. 1989. Bacterial biofilms and surface fouling. Biofouling 1: 323–342.
Marshall KC, R Stout and R Mitchell. 1971. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol 68: 337–348.
May TB and AM Chakrabarty. 1994.Pseudomonas aeruginosa: genes and enzymes of alginate synthesis. Trends Microbiol 2: 151–157.
McCoy WF, JD Bryers, J Robbins and JW Costerton. 1981. Observations in fouling biofilm formation. Can J Microbiol 27: 910–917.
Sutherland IW. 1977. Bacterial polysaccharides—their nature and production. In: Surface Carbohydrates of the Procaryotic Cell (Sutherland IW ed), pp 27–96, Academic Press, London.
van Loosdrecht MCM, J Lyklema, W Norde and AJB Zehnder. 1990. Influence of interfaces on microbial activity. Microbiol Rev 54: 75–87.
Zobell CE. 1943. The effect of solid surfaces upon bacterial activity. J Bacteriol 46: 39–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eginton, P.J., Gibson, H., Holah, J. et al. Quantification of the ease of removal of bacteria from surfaces. Journal of Industrial Microbiology 15, 305–310 (1995). https://doi.org/10.1007/BF01569984
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569984